The mouse dorsal skinfold chamber as a model for the study of thrombolysis by intravital microscopy
- PMID: 22552380
- PMCID: PMC3474710
- DOI: 10.1160/TH11-10-0705
The mouse dorsal skinfold chamber as a model for the study of thrombolysis by intravital microscopy
Abstract
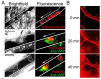
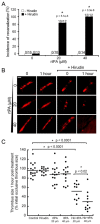
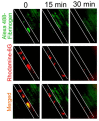
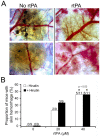
Although intravital microscopy models of thrombosis in mice have contributed to dissect the mechanisms of thrombus formation and stability, they have not been well adapted to study long-term evolution of occlusive thrombi. Here, we assessed the suitability of the dorsal skinfold chamber (DSC) for the study of thrombolysis and testing of thrombolytic agents by intravital microscopy. We show that induction of FeCl3-induced occlusive thrombosis is achievable in microvessels of DSCs, and that thrombi formed in DSCs can be visualised by intravital microscopy using brightfield transmitted light, or fluorescent staining of thrombus components such as fibrinogen, platelets, leukocytes, and von Willebrand factor. Direct application of control saline or recombinant tissue-plasminogen activator (rtPA) to FeCl3-produced thrombi in DSCs did not affect thrombus size or induce recanalisation. However, in the presence of hirudin, rtPA treatment caused a rapid dose-dependent lysis of occlusive thrombi, resulting in recanalisation within 1 hour after treatment. Skin haemorrhage originating from vessels located inside and outside the FeCl3-injured area was also observed in DSCs of rtPA-treated mice. We further show that rtPA-induced thrombolysis was enhanced in plasminogen activator inhibitor-1-deficient (PAI-1-/-) mice, and dropped considerably as the time between occlusion and treatment application increased. Together, our results show that by allowing visualization and measurement of thrombus lysis and potential bleeding complications of thrombolytic treatments, the DSC provides a model for studying endogenous fibrinolysis and for first-line screening of thrombolytic agents. Furthermore, using this system, we found that thrombin and clot aging impair the thrombolytic action of rtPA towards FeCl3-produced thrombi.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
ADAMTS13 exerts a thrombolytic effect in microcirculation.Thromb Haemost. 2012 Sep;108(3):527-32. doi: 10.1160/TH12-01-0046. Epub 2012 Jul 10. Thromb Haemost. 2012. PMID: 22782575 Free PMC article.
-
Thrombolytic and haemorrhagic effects of bolus doses of tissue-type plasminogen activator and a hybrid plasminogen activator with prolonged plasma half-life (K2tu-PA: CGP 42935).Thromb Haemost. 1993 Aug 2;70(2):294-300. Thromb Haemost. 1993. PMID: 8236138
-
Microfluidic Modeling of Thrombolysis.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2626-2637. doi: 10.1161/ATVBAHA.118.311178. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354249
-
Novel and emerging therapies: thrombus-targeted fibrinolysis.Semin Thromb Hemost. 2013 Feb;39(1):48-58. doi: 10.1055/s-0032-1328935. Epub 2012 Oct 3. Semin Thromb Hemost. 2013. PMID: 23034825 Review.
-
Fibrinolytic mechanism, biochemistry, and preclinical pharmacology of recombinant prourokinase.J Vasc Interv Radiol. 1995 Nov-Dec;6(6 Pt 2 Suppl):8S-18S. doi: 10.1016/s1051-0443(95)71242-8. J Vasc Interv Radiol. 1995. PMID: 8770836 Review.
Cited by
-
Role of Neutrophils and NETs in Animal Models of Thrombosis.Int J Mol Sci. 2022 Jan 26;23(3):1411. doi: 10.3390/ijms23031411. Int J Mol Sci. 2022. PMID: 35163333 Free PMC article. Review.
-
In vivo analysis of noise dependent activation of white blood cells and microvascular dysfunction in mice.MethodsX. 2021 Oct 8;8:101540. doi: 10.1016/j.mex.2021.101540. eCollection 2021. MethodsX. 2021. PMID: 34754808 Free PMC article.
-
Dorsal skinfold chamber models in mice.GMS Interdiscip Plast Reconstr Surg DGPW. 2017 Jul 10;6:Doc10. doi: 10.3205/iprs000112. eCollection 2017. GMS Interdiscip Plast Reconstr Surg DGPW. 2017. PMID: 28706772 Free PMC article.
-
Experimental Models to Study Skin Wound Healing with a Focus on Angiogenesis.Med Sci (Basel). 2021 Aug 25;9(3):55. doi: 10.3390/medsci9030055. Med Sci (Basel). 2021. PMID: 34449673 Free PMC article. Review.
-
Thrombus composition and thrombolysis resistance in stroke.Res Pract Thromb Haemost. 2023 May 16;7(4):100178. doi: 10.1016/j.rpth.2023.100178. eCollection 2023 May. Res Pract Thromb Haemost. 2023. PMID: 37538503 Free PMC article.
References
-
- Bizzozero G. Ueber einen neuen Forrnbestandteil des Blutes und dessen Rolle bei der Thrombose und Blutgerinnung. Archiv für pathologische Anatomie und Physiologie und für klinische Medicin. 1882;90:261–332.
-
- Boulaftali Y, Adam F, Venisse L, et al. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood. 2010;115:97–106. - PubMed

