Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split-virion vaccine with mucosal adjuvants show similar levels of cross-protection
- PMID: 22552600
- PMCID: PMC3393367
- DOI: 10.1128/CVI.00016-12
Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split-virion vaccine with mucosal adjuvants show similar levels of cross-protection
Abstract
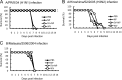
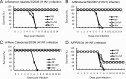
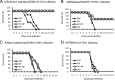
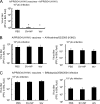
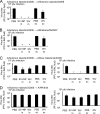
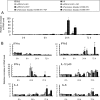
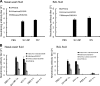
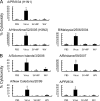
The antigenicity of seasonal human influenza virus changes continuously; thus, a cross-protective influenza vaccine design needs to be established. Intranasal immunization with an influenza split-virion (SV) vaccine and a mucosal adjuvant induces cross-protection; however, no mucosal adjuvant has been assessed clinically. Formalin-inactivated intact human and avian viruses alone (without adjuvant) induce cross-protection against the highly pathogenic H5N1 avian influenza virus. However, it is unknown whether seasonal human influenza formalin-inactivated whole-virion (WV) vaccine alone induces cross-protection against strains within a subtype or in a different subtype of human influenza virus. Furthermore, there are few reports comparing the cross-protective efficacy of the WV vaccine and SV vaccine-mucosal adjuvant mixtures. Here, we found that the intranasal human influenza WV vaccine alone induced both the innate immune response and acquired immune response, resulting in cross-protection against drift variants within a subtype of human influenza virus. The cross-protective efficacy conferred by the WV vaccine in intranasally immunized mice was almost the same as that conferred by a mixture of SV vaccine and adjuvants. The level of cross-protective efficacy was correlated with the cross-reactive neutralizing antibody titer in the nasal wash and bronchoalveolar fluids. However, neither the SV vaccine with adjuvant nor the WV vaccine induced cross-reactive virus-specific cytotoxic T-lymphocyte activity. These results suggest that the intranasal human WV vaccine injection alone is effective against variants within a virus subtype, mainly through a humoral immune response, and that the cross-protection elicited by the WV vaccine and the SV vaccine plus mucosal adjuvants is similar.
Figures

Similar articles
-
An MDCK cell culture-derived formalin-inactivated influenza virus whole-virion vaccine from an influenza virus library confers cross-protective immunity by intranasal administration in mice.Clin Vaccine Immunol. 2013 Jul;20(7):998-1007. doi: 10.1128/CVI.00024-13. Epub 2013 May 1. Clin Vaccine Immunol. 2013. PMID: 23637045 Free PMC article.
-
Intranasal co-administration of 1,8-cineole with influenza vaccine provide cross-protection against influenza virus infection.Phytomedicine. 2017 Oct 15;34:127-135. doi: 10.1016/j.phymed.2017.08.014. Epub 2017 Aug 18. Phytomedicine. 2017. PMID: 28899494
-
Alkyl polyglycoside, a highly promising adjuvant in intranasal split influenza vaccines.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-9. doi: 10.1080/21645515.2016.1278098. Epub 2017 Jan 27. Hum Vaccin Immunother. 2017. PMID: 28129034 Free PMC article.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Intranasal Administration of Whole Inactivated Influenza Virus Vaccine as a Promising Influenza Vaccine Candidate.Viral Immunol. 2017 Jul/Aug;30(6):451-462. doi: 10.1089/vim.2017.0022. Epub 2017 Jun 26. Viral Immunol. 2017. PMID: 28650274 Review.
Cited by
-
An MDCK cell culture-derived formalin-inactivated influenza virus whole-virion vaccine from an influenza virus library confers cross-protective immunity by intranasal administration in mice.Clin Vaccine Immunol. 2013 Jul;20(7):998-1007. doi: 10.1128/CVI.00024-13. Epub 2013 May 1. Clin Vaccine Immunol. 2013. PMID: 23637045 Free PMC article.
-
Development of a universal CTL-based vaccine for influenza.Bioengineered. 2013 Nov-Dec;4(6):374-8. doi: 10.4161/bioe.23573. Epub 2013 Jan 21. Bioengineered. 2013. PMID: 23337287 Free PMC article. Review.
-
Immunogenicity and safety of a novel seasonal influenza preservative-free vaccine manufactured in Kazakhstan: Results of a randomized, comparative, phase II clinical trial in adults.Hum Vaccin Immunother. 2018 Mar 4;14(3):609-614. doi: 10.1080/21645515.2017.1387345. Epub 2017 Dec 12. Hum Vaccin Immunother. 2018. PMID: 29112488 Free PMC article. Clinical Trial.
-
Development of cross-protective influenza a vaccines based on cellular responses.Front Immunol. 2015 May 15;6:237. doi: 10.3389/fimmu.2015.00237. eCollection 2015. Front Immunol. 2015. PMID: 26029218 Free PMC article. Review.
-
Generation of a recombinant temperature-sensitive influenza D virus.Sci Rep. 2023 Mar 7;13(1):3806. doi: 10.1038/s41598-023-30942-z. Sci Rep. 2023. PMID: 36882459 Free PMC article.
References
-
- Agrawal AS, et al. 2010. Genetic characterization of circulating seasonal influenza A viruses (2005–2009) revealed introduction of oseltamivir resistant H1N1 strains during 2009 in eastern India. Infect. Genet. Evol. 10:1188–1198 - PubMed
-
- Akagi T, Kaneko T, Kida T, Akashi M. 2005. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-Phenylalanine as a protein carrier. J. Control Rel. 108:226–236 - PubMed
-
- Amorij JP, Hinrichs W, Frijlink HW, Wilschut JC, Huckriede A. 2010. Needle-free influenza vaccination. Lancet Infect. Dis. 10:699–711 - PubMed
-
- Belshe RB. 2004. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103:177–185 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

