Age effect on bone mineral density changes in breast cancer patients receiving anastrozole: results from the ARBI prospective clinical trial
- PMID: 22552718
- PMCID: PMC3418493
- DOI: 10.1007/s00432-012-1233-z
Age effect on bone mineral density changes in breast cancer patients receiving anastrozole: results from the ARBI prospective clinical trial
Abstract
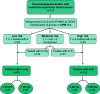
Purpose: We investigated whether age at anastrozole (A) initiation influences the effect of treatment on bone mineral density (BMD). We conducted a post hoc analysis of the dataset of Arimidex Bone Mass Index Oral Bisphosphonates prospective trial, studying the effect of risedronate (R) on BMD of postmenopausal, early breast cancer patients receiving A.
Methods: Patients were stratified into those with normal BMD or mild osteopenia (T > -2) receiving A-only and patients with mild or severe osteopenia (T ≤ -2) or osteoporosis (T < -2.5) receiving A and per os R (A + R). Depending on age on treatment initiation, patients were grouped into two age cohorts, above and below 65 years. BMD change in lumbar spine (LS) and hip (HP) was evaluated at 12 months. An analysis of patients with normal BMD at baseline was additionally performed.
Results: Among patients receiving A-only, women ≤65 years were more likely to have a decrease in LS-BMD than older (p = 0.034). HP-BMD decrease at 12 months was not related to age (p = 0.182). In patients with mild or severe osteopenia or osteoporosis, treated with A + R, no age effect was observed for LS or HP (p = 0.099 and p = 0.939, respectively). Among patients with normal BMD at baseline, the age effect on LS-BMD change was more profound (p = 0.026).
Conclusions: Our study suggests that younger postmenopausal women with normal BMD or mild osteopenia receiving A-only face an increased risk of bone loss in LS. Among patients with mild or severe osteopenia or osteoporosis treated with A + R, 12 months LS or HP BMD variations were configured regardless of age group.
Conflict of interest statement
CM has received educational grants and lecture honoraria from AstraZeneca, Novartis (Basel, Switzerland), and Pfizer Inc (New York, NY, USA). ET, BV, GX, VZ, JM, DK, and HG have received unrestricted educational grants from AstraZeneca, Novartis, and Pfizer Inc.
Figures


References
-
- Aapro M, Abrahamsson PA, Body JJ et al (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 19:420–432 - PubMed
-
- Ashcroft AJ, Davies FE, Morgan GJ (2003) Aetiology of bone disease and the role of bisphosphonates in multiple myeloma. Lancet Oncol 4:284–292 - PubMed
-
- Baum M, Buzdar A, Cuzick J et al (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98:1802–1810 - PubMed
-
- Bjornerem A, Straume B, Midtby M et al (2004) Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso study. J Clin Endocrinol Metab 89:6039–6047 - PubMed
-
- Body JJ, Bartl R, Burckhardt P et al (1998) Current use of bisphosphonates in oncology. International Bone and Cancer Study Group. J Clin Oncol 16:3890–3899 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

