Mixing method affects elution and strength of high-dose ALBC: a pilot study
- PMID: 22552766
- PMCID: PMC3442001
- DOI: 10.1007/s11999-012-2351-2
Mixing method affects elution and strength of high-dose ALBC: a pilot study
Abstract
Background: High-dose antimicrobial-loaded bone cement (ALBC) is used to treat orthopaedic infections. High-dose ALBC is not commercially available and requires surgeon directed formulation, and there are several different methods used to mix high-dose ALBC.
Questions/purposes: We asked whether the mixing method affected antimicrobial elution and mechanical properties of high-dose ALBC.
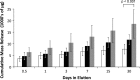
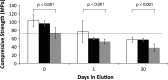
Methods: ALBC was formulated with Simplex P bone cement and 10 g of vancomycin per batch using one of three mixing methods: (1) hand-stirred using a standard bowl and spatula, (2) bowl-mixed using a mechanical mixing bowl, and (3) dough-phase mixing where the vancomycin was left in chunks (1-5 mm) and folded into the cement during the dough phase after adding the monomer. We eluted 45 standardized test cylinders (15 per mixing technique) for 30 days under infinite sink conditions. We tested 135 (45 per mixing method) similarly eluted cylinders in axial compression to failure.
Results: Dough-phase mixing lead to greater antimicrobial delivery, but lower compressive strength than the hand-stirred or bowl-mixed methods. Dough-phase cement released 18,570 lg of vancomycin versus 11,731 for hand-stirred and 7700 μg for bowl mixed. Compressive strength for dough-phase mixing after 30 days of elution was 36 MPa, while both hand-stirred and bowl mixed cements were 56 MPa.
Conclusions: Performance of high-dose ALBC was affected by mixing method. Dough-phase mixing led to greater antimicrobial delivery, but caused greater loss in compressive strength.
Figures

References
-
- Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. - PubMed
-
- American Society for Testing And Materials, Subcommittee F04.11. ASTM F451-08 Standard Specification for Acrylic Bone Cement. 2008.13.01. Available at: http://www.astm.org/Standards/F451.htm. Accessed December 19, 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

