Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway
- PMID: 22552773
- PMCID: PMC3493190
- DOI: 10.1093/toxsci/kfs164
Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway
Abstract
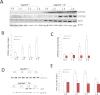
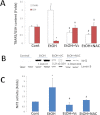
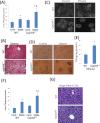
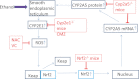
Chronic ethanol consumption was previously shown to induce CYP2A5 in mice, and this induction of CYP2A5 by ethanol was CYP2E1 dependent. In this study, the mechanisms of CYP2E1-dependent ethanol induction of CYP2A5 were investigated. CYP2E1 was induced by chronic ethanol consumption to the same degree in wild-type (WT) mice and CYP2A5 knockout (Cyp2a5 (-/-)) mice, suggesting that unlike the CYP2E1-dependent ethanol induction of CYP2A5, ethanol induction of CYP2E1 is not CYP2A5 dependent. Microsomal ethanol oxidation was about 25% lower in Cyp2a5 (-/-) mice compared with that in WT mice, suggesting that CYP2A5 can oxidize ethanol although to a lesser extent than CYP2E1 does. CYP2A5 was induced by short-term ethanol consumption in human CYP2E1 transgenic knockin (Cyp2e1 (-/-) KI) mice but not in CYP2E1 knockout (Cyp2e1 (-/-)) mice. The redox-sensitive transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) was also induced by acute ethanol in Cyp2e1 (-/-) KI mice but not in Cyp2e1 (-/-) mice. Ethanol induction of CYP2A5 in Nrf2 knockout (Nrf2 (-/-)) mice was lower compared with that in WT mice, whereas CYP2E1 induction by ethanol was comparable in WT and Nrf2 (-/-) mice. Antioxidants (N-acetyl-cysteine and vitamin C), which blocked oxidative stress induced by chronic ethanol in WT mice and acute ethanol in Cyp2e1 (-/-) KI mice, also blunted the induction of CYP2A5 and Nrf2 by ethanol but not the induction of CYP2E1 by ethanol. These results suggest that oxidative stress induced by ethanol via induction of CYP2E1 upregulates Nrf2 activity, which in turn regulates ethanol induction of CYP2A5. Results obtained from primary hepatocytes, mice gavaged with binge ethanol or fed chronic ethanol, show that Nrf2-regulated ethanol induction of CYP2A5 protects against ethanol-induced steatosis.
Figures





References
-
- Abu-Bakar A., Arthur D. M., Aganovic S., Ng J. C., Lang M. A. 2011. Inducible bilirubin oxidase: A novel function for the mouse cytochrome P450 2A5 Toxicol. Appl. Pharmacol 257 14–22 - PubMed
-
- Abu-Bakar A., Lamsa V., Arpainen S., Moore M. R., Lang M. A., Hakkola J. 2007. Regulation of CYP2A5 gene by the transcription factor nuclear factor (erythroid-derived 2)-like 2 Drug Metab. Disp 35 787–794 - PubMed
-
- Abu-Bakar A., Moore M. R., Lang M. A. 2005. Evidence for induced microsomal bilirubin degradation by cytochrome P450 2A5 Biochem. Pharmacol 70 1527–1535 - PubMed
-
- Abu-Bakar A., Satarug S., Marks G. C., Lang M. A., Moore M. R. 2004. Acute cadmium chloride administration induces hepatic and renal CYP2A5 mRNA, protein and activity in the mouse: Involvement of transcription factor NRF2 Toxicol. Lett 148 199–210 - PubMed
-
- Arpiainen S., Järvenpää S. M., Manninen A., Viitala P., Lang M. A., Pelkonen O, Hakkola J. 2008. Coactivator PGC-1alpha regulates the fasting inducible xenobiotic-metabolizing enzyme CYP2A5 in mouse primary hepatocytes Toxicol. Appl. Pharmacol 232 135–141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

