Nitric oxide-donating aspirin induces G2/M phase cell cycle arrest in human cancer cells by regulating phase transition proteins
- PMID: 22552812
- PMCID: PMC4430830
- DOI: 10.3892/ijo.2012.1455
Nitric oxide-donating aspirin induces G2/M phase cell cycle arrest in human cancer cells by regulating phase transition proteins
Abstract
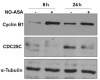
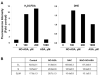
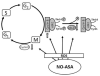
NO-aspirin (NO-ASA), consisting of aspirin and a nitric oxide-releasing group, is safer than aspirin and effective in colon cancer prevention. Here, we examined the mechanism of action of NO-ASA by focusing primarily on its effects on the cell cycle. NO-ASA reduced the growth of several cell lines from colon, pancreas, skin, cervix and breast cancer much more potently than aspirin, with 24-h IC(50) values of 133-268 µM, while those of ASA were >1,000 µM. NO-ASA elevated the intracellular levels of reactive oxygen species, generating a state of oxidative stress. In all cell lines examined, NO-ASA induced cell cycle arrest in the G(2)/M phase transition accompanied by altered expression of G(2)/M transition-related proteins. In SW480 colon cancer cells NO-ASA modulated proteins controlling this transition. Thus, it markedly increased the levels of cyclin B1, decreased the expression of cyclin D1 and Cdc25C, and increased the Thr14/Tyr15-phosphorylation of Cdk1 while leaving unchanged its protein levels. These changes, including the G2/M arrest, were prevented by pretreating the cells with the anti-oxidant N-acetyl-cysteine, indicating that redox signaling is likely responsible for the cell cycle changes, a conclusion consistent with the known redox regulation of these proteins. Collectively, these results confirm the profound cytokinetic effect of NO-ASA and provide strong evidence that it regulates cell cycle transitions through its ability to induce oxidative stress, which activates redox signaling in the target cell.
Figures


References
-
- American Cancer Society . Cancer Facts and Figures 2011. American Cancer Society; Atlanta, GA: 2011.
-
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. - PubMed
-
- Rayyan Y, Williams J, Rigas B. The role of NSAIDs in the prevention of colon cancer. Cancer Invest. 2002;20:1002–1011. - PubMed
-
- Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. - PubMed
-
- Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med. 2004;10:324–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

