Phosphate homeostasis and its role in bone health
- PMID: 22552885
- PMCID: PMC3461213
- DOI: 10.1007/s00467-012-2175-z
Phosphate homeostasis and its role in bone health
Erratum in
-
Erratum to: Phosphate homeostasis and its role in bone health.Pediatr Nephrol. 2017 Oct;32(10):1999. doi: 10.1007/s00467-017-3713-5. Pediatr Nephrol. 2017. PMID: 28685172 Free PMC article. No abstract available.
Abstract
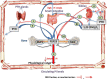
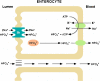
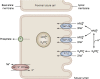
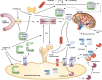
Phosphate is one of the most abundant minerals in the body, and its serum levels are regulated by a complex set of processes occurring in the intestine, skeleton, and kidneys. The currently known main regulators of phosphate homeostasis include parathyroid hormone (PTH), calcitriol, and a number of peptides collectively known as the "phosphatonins" of which fibroblast growth factor-23 (FGF-23) has been best defined. Maintenance of extracellular and intracellular phosphate levels within a narrow range is important for many biological processes, including energy metabolism, cell signaling, regulation of protein synthesis, skeletal development, and bone integrity. The presence of adequate amounts of phosphate is critical for the process of apoptosis of mature chondrocytes in the growth plate. Without the presence of this mineral in high enough quantities, chondrocytes will not go into apoptosis, and the normal physiological chain of events that includes invasion of blood vessels and the generation of new bone will be blocked, resulting in rickets and delayed growth. In the rest of the skeleton, hypophosphatemia will result in osteomalacia due to an insufficient formation of hydroxyapatite. This review will address phosphate metabolism and its role in bone health.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

