Modulation of bladder function by luminal adenosine turnover and A1 receptor activation
- PMID: 22552934
- PMCID: PMC3404591
- DOI: 10.1152/ajprenal.00566.2011
Modulation of bladder function by luminal adenosine turnover and A1 receptor activation
Abstract
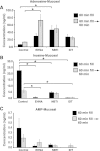
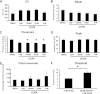
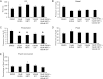
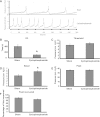
The bladder uroepithelium transmits information to the underlying nervous and musculature systems, is under constant cyclical strain, expresses all four adenosine receptors (A(1), A(2A), A(2B), and A(3)), and is a site of adenosine production. Although adenosine has a well-described protective effect in several organs, there is a lack of information about adenosine turnover in the uroepithelium or whether altering luminal adenosine concentrations impacts bladder function or overactivity. We observed that the concentration of extracellular adenosine at the mucosal surface of the uroepithelium was regulated by ecto-adenosine deaminase and by equilibrative nucleoside transporters, whereas adenosine kinase and equilibrative nucleoside transporters modulated serosal levels. We further observed that enriching endogenous adenosine by blocking its routes of metabolism or direct activation of mucosal A(1) receptors with 2-chloro-N(6)-cyclopentyladenosine (CCPA), a selective agonist, stimulated bladder activity by lowering the threshold pressure for voiding. Finally, CCPA did not quell bladder hyperactivity in animals with acute cyclophosphamide-induced cystitis but instead exacerbated their irritated bladder phenotype. In conclusion, we find that adenosine levels at both surfaces of the uroepithelium are modulated by turnover, that blocking these pathways or stimulating A(1) receptors directly at the luminal surface promotes bladder contractions, and that adenosine further stimulates voiding in animals with cyclophosphamide-induced cystitis.
Figures



Similar articles
-
Activation of the adenosine A1 receptor in the lumbosacral spinal cord improves bladder overactivity in rats with cystitis induced by cyclophosphamide.Int Urol Nephrol. 2023 Sep;55(9):2183-2191. doi: 10.1007/s11255-023-03659-1. Epub 2023 Jun 18. Int Urol Nephrol. 2023. PMID: 37330931
-
Suppression of adenosine A2a receptors alleviates bladder overactivity and hyperalgesia in cyclophosphamide-induced cystitis by inhibiting TRPV1.Biochem Pharmacol. 2021 Jan;183:114340. doi: 10.1016/j.bcp.2020.114340. Epub 2020 Nov 13. Biochem Pharmacol. 2021. PMID: 33189675
-
Adenosine receptor expression and function in bladder uroepithelium.Am J Physiol Cell Physiol. 2006 Aug;291(2):C254-65. doi: 10.1152/ajpcell.00025.2006. Epub 2006 Mar 29. Am J Physiol Cell Physiol. 2006. PMID: 16571869
-
The uroepithelial-associated sensory web.Kidney Int. 2007 Nov;72(9):1057-64. doi: 10.1038/sj.ki.5002439. Epub 2007 Aug 1. Kidney Int. 2007. PMID: 17667988 Review.
-
The role of the adenosinergic system in lung fibrosis.Pharmacol Res. 2013 Oct;76:182-9. doi: 10.1016/j.phrs.2013.08.004. Epub 2013 Aug 28. Pharmacol Res. 2013. PMID: 23994158 Review.
Cited by
-
Increased urothelial paracellular transport promotes cystitis.Am J Physiol Renal Physiol. 2015 Dec 15;309(12):F1070-81. doi: 10.1152/ajprenal.00200.2015. Epub 2015 Sep 30. Am J Physiol Renal Physiol. 2015. PMID: 26423859 Free PMC article.
-
Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria.Int J Mol Sci. 2023 Apr 15;24(8):7322. doi: 10.3390/ijms24087322. Int J Mol Sci. 2023. PMID: 37108490 Free PMC article.
-
RAB27B requirement for stretch-induced exocytosis in bladder umbrella cells.Am J Physiol Cell Physiol. 2018 Mar 1;314(3):C349-C365. doi: 10.1152/ajpcell.00218.2017. Epub 2017 Nov 22. Am J Physiol Cell Physiol. 2018. PMID: 29167152 Free PMC article.
-
Urothelial purine release during filling of murine and primate bladders.Am J Physiol Renal Physiol. 2016 Oct 1;311(4):F708-F716. doi: 10.1152/ajprenal.00387.2016. Epub 2016 Jul 27. Am J Physiol Renal Physiol. 2016. PMID: 27465992 Free PMC article.
-
A1 Adenosine Receptor-Mediated Inhibition of Parasympathetic Neuromuscular Transmission in Human and Murine Urinary Bladder.J Pharmacol Exp Ther. 2016 Jan;356(1):116-22. doi: 10.1124/jpet.115.228882. Epub 2015 Nov 3. J Pharmacol Exp Ther. 2016. PMID: 26534943 Free PMC article.
References
-
- Apodaca G. The uroepithelium: not just a passive barrier. Traffic 5: 117–128, 2004 - PubMed
-
- Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 - PubMed
-
- Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107: 603–613, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

