The abbreviated pluripotent cell cycle
- PMID: 22552993
- PMCID: PMC3667593
- DOI: 10.1002/jcp.24104
The abbreviated pluripotent cell cycle
Abstract
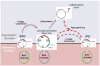
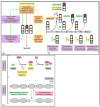
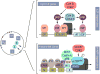
Human embryonic stem cells (hESCs) and induced pluripotent stem cells proliferate rapidly and divide symmetrically producing equivalent progeny cells. In contrast, lineage committed cells acquire an extended symmetrical cell cycle. Self-renewal of tissue-specific stem cells is sustained by asymmetric cell division where one progeny cell remains a progenitor while the partner progeny cell exits the cell cycle and differentiates. There are three principal contexts for considering the operation and regulation of the pluripotent cell cycle: temporal, regulatory, and structural. The primary temporal context that the pluripotent self-renewal cell cycle of hESCs is a short G1 period without reducing periods of time allocated to S phase, G2, and mitosis. The rules that govern proliferation in hESCs remain to be comprehensively established. However, several lines of evidence suggest a key role for the naïve transcriptome of hESCs, which is competent to stringently regulate the embryonic stem cell (ESC) cell cycle. This supports the requirements of pluripotent cells to self-propagate while suppressing expression of genes that confer lineage commitment and/or tissue specificity. However, for the first time, we consider unique dimensions to the architectural organization and assembly of regulatory machinery for gene expression in nuclear microenviornments that define parameters of pluripotency. From both fundamental biological and clinical perspectives, understanding control of the abbreviated ESC cycle can provide options to coordinate control of proliferation versus differentiation. Wound healing, tissue engineering, and cell-based therapy to mitigate developmental aberrations illustrate applications that benefit from knowledge of the biology of the pluripotent cell cycle.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


References
-
- Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, Izpisua Belmonte JC. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13:652–659. - PubMed
-
- Amente S, Bertoni A, Morano A, Lania L, Avvedimento EV, Majello B. LSD1-mediated demethylation of histone H3 lysine 4 triggers Myc-induced transcription. Oncogene. 2010a;29:3691–3702. - PubMed
-
- Amente S, Lania L, Avvedimento EV, Majello B. DNA oxidation drives Myc mediated transcription. Cell Cycle. 2010b;9:3002–3004. - PubMed
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

