Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain
- PMID: 22553021
- PMCID: PMC3752044
- DOI: 10.1523/JNEUROSCI.6343-11.2012
Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain
Abstract
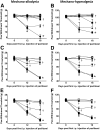
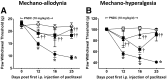
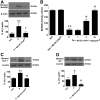
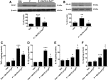
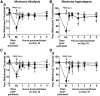
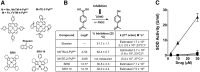
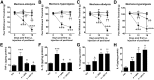
Chemotherapy-induced peripheral neuropathy (CIPN) accompanied by chronic neuropathic pain is a major dose-limiting side effect of a large number of antitumoral agents including paclitaxel (Taxol). Thus, CIPN is one of most common causes of dose reduction and discontinuation of what is otherwise a life-saving therapy. Neuropathological changes in spinal cord are linked to CIPN, but the causative mediators and mechanisms remain poorly understood. We report that formation of peroxynitrite (PN) in response to activation of nitric oxide synthases and NADPH oxidase in spinal cord contributes to neuropathological changes through two mechanisms. The first involves modulation of neuroexcitatory and proinflammatory (TNF-α and IL-1β) and anti-inflammatory (IL-10 and IL-4) cytokines in favor of the former. The second involves post-translational nitration and modification of glia-derived proteins known to be involved in glutamatergic neurotransmission (astrocyte-restricted glutamate transporters and glutamine synthetase). Targeting PN with PN decomposition catalysts (PNDCs) not only blocked the development of paclitaxel-induced neuropathic pain without interfering with antitumor effects, but also reversed it once established. Herein, we describe our mechanistic study on the role(s) of PN and the prevention of neuropathic pain in rats using known PNDCs (FeTMPyP(5+) and MnTE-2-PyP(5+)). We also demonstrate the prevention of CIPN with our two new orally active PNDCs, SRI6 and SRI110. The improved chemical design of SRI6 and SRI110 also affords selectivity for PN over other reactive oxygen species (such as superoxide). Our findings identify PN as a critical determinant of CIPN, while providing the rationale toward development of superoxide-sparing and "PN-targeted" therapeutics.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
