Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex
- PMID: 22553025
- PMCID: PMC3526954
- DOI: 10.1523/JNEUROSCI.0343-12.2012
Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex
Abstract
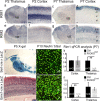
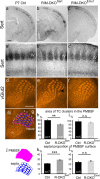
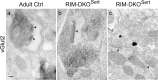
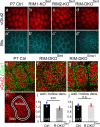
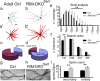
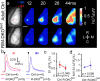
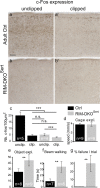
To assess the impact of synaptic neurotransmitter release on neural circuit development, we analyzed barrel cortex formation after thalamic or cortical ablation of RIM1 and RIM2 proteins, which control synaptic vesicle fusion. Thalamus-specific deletion of RIMs reduced neurotransmission efficacy by 67%. A barrelless phenotype was found with a dissociation of effects on the presynaptic and postsynaptic cellular elements of the barrel. Presynaptically, thalamocortical axons formed a normal whisker map, whereas postsynaptically the cytoarchitecture of layer IV neurons was altered as spiny stellate neurons were evenly distributed and their dendritic trees were symmetric. Strikingly, cortex-specific deletion of the RIM genes did not modify barrel development. Adult mice with thalamic-specific RIM deletion showed a lack of activity-triggered immediate early gene expression and altered sensory-related behaviors. Thus, efficient synaptic release is required at thalamocortical but not at corticocortical synapses for building the whisker to barrel map and for efficient sensory function.
Figures

Similar articles
-
Sensory experience restructures thalamocortical axons during adulthood.Neuron. 2012 May 24;74(4):648-55. doi: 10.1016/j.neuron.2012.03.022. Neuron. 2012. PMID: 22632723 Free PMC article.
-
Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons.J Neurosci. 2002 Nov 1;22(21):9171-5. doi: 10.1523/JNEUROSCI.22-21-09171.2002. J Neurosci. 2002. PMID: 12417641 Free PMC article.
-
Area-Specific Synapse Structure in Branched Posterior Nucleus Axons Reveals a New Level of Complexity in Thalamocortical Networks.J Neurosci. 2020 Mar 25;40(13):2663-2679. doi: 10.1523/JNEUROSCI.2886-19.2020. Epub 2020 Feb 13. J Neurosci. 2020. PMID: 32054677 Free PMC article.
-
Thalamocortical and corticocortical interactions in the somatosensory system.Prog Brain Res. 2001;130:90-110. Prog Brain Res. 2001. PMID: 11480292 Review. No abstract available.
-
[Research progress on barrel cortex and its plasticity].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011 May;40(3):332-7. doi: 10.3785/j.issn.1008-9292.2011.03.018. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 21671497 Review. Chinese.
Cited by
-
How the Barrel Cortex Became a Working Model for Developmental Plasticity: A Historical Perspective.J Neurosci. 2020 Aug 19;40(34):6460-6473. doi: 10.1523/JNEUROSCI.0582-20.2020. J Neurosci. 2020. PMID: 32817388 Free PMC article.
-
Thalamocortical axons control the cytoarchitecture of neocortical layers by area-specific supply of VGF.Elife. 2022 Mar 15;11:e67549. doi: 10.7554/eLife.67549. Elife. 2022. PMID: 35289744 Free PMC article.
-
Serotonin, birth, and thalamocortical wiring.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2312515120. doi: 10.1073/pnas.2312515120. Epub 2023 Aug 31. Proc Natl Acad Sci U S A. 2023. PMID: 37651446 Free PMC article. No abstract available.
-
FGF-FGFR Mediates the Activity-Dependent Dendritogenesis of Layer IV Neurons during Barrel Formation.J Neurosci. 2017 Dec 13;37(50):12094-12105. doi: 10.1523/JNEUROSCI.1174-17.2017. Epub 2017 Nov 2. J Neurosci. 2017. PMID: 29097598 Free PMC article.
-
Early-life maturation of the somatosensory cortex: sensory experience and beyond.Front Neural Circuits. 2024 Jul 8;18:1430783. doi: 10.3389/fncir.2024.1430783. eCollection 2024. Front Neural Circuits. 2024. PMID: 39040685 Free PMC article. Review.
References
-
- Benshalom G, White EL. Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. J Comp Neurol. 1986;253:303–314. - PubMed
-
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. - PubMed
-
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
-
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
