Stimulus site and modality dependence of functional activity within the human spinal cord
- PMID: 22553029
- PMCID: PMC6622146
- DOI: 10.1523/JNEUROSCI.2543-11.2012
Stimulus site and modality dependence of functional activity within the human spinal cord
Abstract
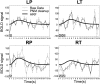
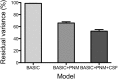
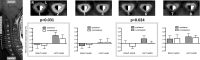
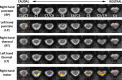
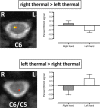
Chronic pain is thought to arise because of maladaptive changes occurring within the peripheral nervous system and CNS. The transition from acute to chronic pain is known to involve the spinal cord (Woolf and Salter, 2000). Therefore, to investigate altered human spinal cord function and translate results obtained from other species, a noninvasive neuroimaging technique is desirable. We have investigated the functional response in the cervical spinal cord of 18 healthy human subjects (aged 22-40 years) to noxious thermal and non-noxious tactile stimulation of the left and right forearms. Physiological noise, which is a significant source of signal variability in the spinal cord, was accounted for in the general linear model. Group analysis, performed using a mixed-effects model, revealed distinct regions of activity that were dependent on both the side and the type of stimulation. In particular, thermal stimulation on the medial aspect of the wrist produced activity within the C6/C5 segment ipsilateral to the side of stimulation. Similar to data recorded in animals (Fitzgerald, 1982), painful thermal stimuli produced increased ipsilateral and decreased contralateral blood flow, which may reflect, respectively, excitatory and inhibitory processes. Nonpainful punctate stimulation of the thenar eminence provoked more diffuse activity but was still ipsilateral to the side of stimulation. These results present the first noninvasive evidence for a lateralized response to noxious and non-noxious stimuli in the human spinal cord. The development of these techniques opens the path to understanding, at a subject-specific level, central sensitization processes that contribute to chronic pain states.
Figures


References
-
- Agosta F, Valsasina P, Rocca MA, Caputo D, Sala S, Judica E, Stroman PW, Filippi M. Evidence for enhanced functional activity of cervical cord in relapsing multiple sclerosis. Magn Reson Med. 2008;59:1035–1042. - PubMed
-
- Bouwman CJ, Wilmink JT, Mess WH, Backes WH. Spinal cord functional MRI at 3 T: gradient echo echo-planar imaging versus turbo spin echo. Neuroimage. 2008;43:288–296. - PubMed
-
- Brooks JC, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, Jenkinson M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage. 2008;39:680–692. - PubMed
-
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
