Connectivity-based parcellation of the human orbitofrontal cortex
- PMID: 22553030
- PMCID: PMC6622144
- DOI: 10.1523/JNEUROSCI.0257-12.2012
Connectivity-based parcellation of the human orbitofrontal cortex
Abstract
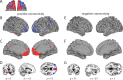
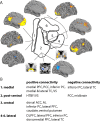
The primate orbitofrontal cortex (OFC) is involved in reward processing, learning, and decision making. Research in monkeys has shown that this region is densely connected with higher sensory, limbic, and subcortical regions. Moreover, a parcellation of the monkey OFC into two subdivisions has been suggested based on its intrinsic anatomical connections. However, in humans, little is known about any functional subdivisions of the OFC except for a rather coarse medial/lateral distinction. Here, we used resting-state fMRI in combination with unsupervised clustering techniques to investigate whether OFC subdivisions can be revealed based on their functional connectivity profiles with other brain regions. Examination of different cluster solutions provided support for a parcellation into two parts as observed in monkeys, but it also highlighted a much finer hierarchical clustering of the orbital surface. Specifically, we identified (1) a medial, (2) a posterior-central, (3) a central, and (4-6) three lateral clusters spanning the anterior-posterior gradient. Consistent with animal tracing studies, these OFC clusters were connected to other cortical regions such as prefrontal, temporal, and parietal cortices but also subcortical areas in the striatum and the midbrain. These connectivity patterns provide important implications for identifying specific functional roles of OFC subdivisions for reward processing, learning, and decision making. Moreover, this parcellation schema can provide guidance to report results in future studies.
Figures





References
-
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. - PubMed
-
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. - PubMed
-
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
