Inhibitory control of synaptic and behavioral plasticity by octopaminergic signaling
- PMID: 22553037
- PMCID: PMC3371232
- DOI: 10.1523/JNEUROSCI.6517-11.2012
Inhibitory control of synaptic and behavioral plasticity by octopaminergic signaling
Abstract
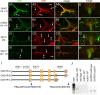
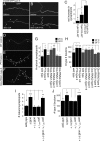
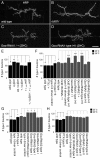
Adrenergic receptors and their ligands are important regulators of synaptic plasticity and metaplasticity, but the exact mechanisms underlying their action are still poorly understood. Octopamine, the invertebrate homolog of mammalian adrenaline or noradrenaline, plays important roles in modulating behavior and synaptic functions. We previously uncovered an octopaminergic positive-feedback mechanism to regulate structural synaptic plasticity during development and in response to starvation. Under this mechanism, activation of Octß2R autoreceptors by octopamine at octopaminergic neurons initiated a cAMP-dependent cascade that stimulated the development of new synaptic boutons at the Drosophila larval neuromuscular junction (NMJ). However, the regulatory mechanisms that served to brake such positive feedback were not known. Here, we report the presence of an alternative octopamine autoreceptor, Octß1R, with antagonistic functions on synaptic growth. Mutations in octß1r result in the overgrowth of both glutamatergic and octopaminergic NMJs, suggesting that Octß1R is a negative regulator of synaptic expansion. As Octß2R, Octß1R functioned in a cell-autonomous manner at presynaptic motorneurons. However, unlike Octß2R, which activated a cAMP pathway, Octß1R likely inhibited cAMP production through inhibitory Goα. Despite its inhibitory role, Octß1R was required for acute changes in synaptic structure in response to octopamine and for starvation-induced increase in locomotor speed. These results demonstrate the dual action of octopamine on synaptic growth and behavioral plasticity, and highlight the important role of inhibitory influences for normal responses to physiological stimuli.
Figures




References
-
- Anholt RR, Mackay TF. Quantitative genetic analyses of complex behaviours in Drosophila. Nat Rev Genet. 2004;5:838–849. - PubMed
-
- Balfanz S, Strünker T, Frings S, Baumann A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J Neurochem. 2005;93:440–451. - PubMed
-
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
