Duration tuning across vertebrates
- PMID: 22553042
- PMCID: PMC6622122
- DOI: 10.1523/JNEUROSCI.5624-11.2012
Duration tuning across vertebrates
Abstract
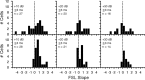
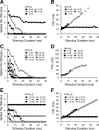
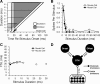
Signal duration is important for identifying sound sources and determining signal meaning. Duration-tuned neurons (DTNs) respond preferentially to a range of stimulus durations and maximally to a best duration (BD). Duration-tuned neurons are found in the auditory midbrain of many vertebrates, although studied most extensively in bats. Studies of DTNs across vertebrates have identified cells with BDs and temporal response bandwidths that mirror the range of species-specific vocalizations. Neural tuning to stimulus duration appears to be universal among hearing vertebrates. Herein, we test the hypothesis that neural mechanisms underlying duration selectivity may be similar across vertebrates. We instantiated theoretical mechanisms of duration tuning in computational models to systematically explore the roles of excitatory and inhibitory receptor strengths, input latencies, and membrane time constant on duration tuning response profiles. We demonstrate that models of duration tuning with similar neural circuitry can be tuned with species-specific parameters to reproduce the responses of in vivo DTNs from the auditory midbrain. To relate and validate model output to in vivo responses, we collected electrophysiological data from the inferior colliculus of the awake big brown bat, Eptesicus fuscus, and present similar in vivo data from the published literature on DTNs in rats, mice, and frogs. Our results support the hypothesis that neural mechanisms of duration tuning may be shared across vertebrates despite species-specific differences in duration selectivity. Finally, we discuss how the underlying mechanisms of duration selectivity relate to other auditory feature detectors arising from the interaction of neural excitation and inhibition.
Figures









References
-
- Brand A, Urban R, Grothe B. Duration tuning in the mouse auditory midbrain. J Neurophysiol. 2000;84:1790–1799. - PubMed
-
- Brugge JF. An overview of central auditory processing. In: Popper AN, Fay RR, editors. The mammalian auditory pathway: neurophysiology. New York: Springer; 1992. pp. 1–33.
-
- Burger RM, Pollak GD. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. J Neurophysiol. 1998;80:1686–1701. - PubMed
-
- Carnevale NT, Hines ML. The NEURON book. Cambridge, UK: Cambridge UP; 2006.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
