Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk?
- PMID: 22553145
- PMCID: PMC3427719
- DOI: 10.1002/ijc.27623
Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk?
Abstract
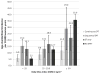
Given the strong link between use of unopposed estrogens and development of endometrial cancers, estrogens are usually prescribed with a progestin, particularly for women with intact uteri. Some studies suggest that sequential use of progestins may increase risk; however, the moderating effects of usage patterns or patient characteristics, including body mass index (BMI), are unknown. We evaluated menopausal hormone use and incident endometrial cancer (n = 885) in 68,419 postmenopausal women with intact uteri enrolled in the National Institutes of Health-American Association of Retired Persons Diet and Health study. Participants completed a risk factor questionnaire in 1996-1997 and were followed up through 2006. Hazard rate ratios (RRs) and 95% confidence intervals (CIs) were estimated using Cox regression. Among 19,131 women reporting exclusive estrogen plus progestin use, 176 developed endometrial cancer (RR = 0.88; 95% CI = 0.74-1.06). Long-duration (≥ 10 years) sequential (<15 days progestin per month) estrogen plus progestin use was positively associated with risk (RR = 1.88; 95% CI = 1.36-2.60], whereas continuous (>25 days progestin per month) estrogen plus progestin use was associated with a decreased risk (RR = 0.64; 95% CI = 0.49-0.83). Increased risk for sequential estrogen plus progestin was seen only among thin-to-normal weight women (BMI < 25 kg/m(2); RR = 2.53). Our findings support that specific categories of estrogen plus progestin use increases endometrial cancer risk, specifically long durations of sequential progestins, whereas decreased endometrial cancer risk was observed for users of short-duration continuous progestins. Risks were highest among thin-to-normal weight women, presumably reflecting their lower endogenous estrogen levels, suggesting that menopausal hormones and obesity increase endometrial cancer through common etiologic pathways.
Copyright © 2012 UICC.
Figures
References
-
- Reis L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. 2010.
-
- Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–14. - PubMed
-
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–7. - PubMed
-
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–70. - PubMed
-
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

