Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection
- PMID: 22553324
- PMCID: PMC3416327
- DOI: 10.1128/JVI.00164-12
Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection
Abstract
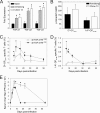
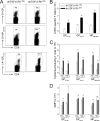
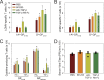
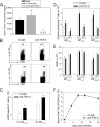
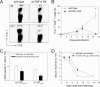
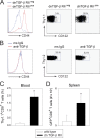
Persistent viral infections often overburden the immune system and are a major cause of disease in humans. During many persistent infections, antiviral T cells are maintained in a state of immune exhaustion characterized by diminished effector and helper functions. In mammalian systems, an extensive immune regulatory network exists to limit unwanted, potentially fatal immunopathology by inducing T cell exhaustion. However, this regulatory network at times overprotects the host and fosters viral persistence by severely dampening adaptive immune responsiveness. Importantly, recent studies have shown that T cell exhaustion is mediated in part by host immunoregulatory pathways (e.g., programmed death 1 [PD-1], interleukin 10 [IL-10]) and that therapeutic blockade of these pathways either before or during persistent infection can promote viral clearance. Transforming growth factor beta (TGF-β) is another immunosuppressive cytokine known to impede both self- and tumor-specific T cells, but its role in regulating antiviral immunity is not entirely understood. In this study, we inhibited TGF-β with three potent antagonists to determine whether neutralization of this regulatory molecule is a viable approach to control a persistent viral infection. Our results revealed that these inhibitors modestly elevate the number of antiviral T cells following infection with a persistent variant of lymphocytic choriomeningitis virus (LCMV) but have no impact on viral clearance. These data suggest that therapeutic neutralization of TGF-β is not an efficacious means to promote clearance of a persistent viral infection.
Figures
Similar articles
-
Therapeutic depletion of natural killer cells controls persistent infection.J Virol. 2014 Feb;88(4):1953-60. doi: 10.1128/JVI.03002-13. Epub 2013 Nov 27. J Virol. 2014. PMID: 24284324 Free PMC article.
-
PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells.J Clin Invest. 2013 Jun;123(6):2604-15. doi: 10.1172/JCI67008. Epub 2013 May 15. J Clin Invest. 2013. PMID: 23676462 Free PMC article.
-
Prevention of CD8 T Cell Deletion during Chronic Viral Infection.Viruses. 2021 Jun 22;13(7):1189. doi: 10.3390/v13071189. Viruses. 2021. PMID: 34206262 Free PMC article.
-
Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus.Viruses. 2019 Feb 13;11(2):156. doi: 10.3390/v11020156. Viruses. 2019. PMID: 30781904 Free PMC article. Review.
-
TNFRs and Control of Chronic LCMV Infection: Implications for Therapy.Trends Immunol. 2015 Nov;36(11):697-708. doi: 10.1016/j.it.2015.09.005. Epub 2015 Oct 17. Trends Immunol. 2015. PMID: 26481667 Review.
Cited by
-
Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review.
-
Targeting Transcriptional Regulators of CD8+ T Cell Dysfunction to Boost Anti-Tumor Immunity.Vaccines (Basel). 2015 Sep 17;3(3):771-802. doi: 10.3390/vaccines3030771. Vaccines (Basel). 2015. PMID: 26393659 Free PMC article. Review.
-
TGF-β receptor maintains CD4 T helper cell identity during chronic viral infections.J Clin Invest. 2016 Oct 3;126(10):3799-3813. doi: 10.1172/JCI87041. Epub 2016 Sep 6. J Clin Invest. 2016. PMID: 27599295 Free PMC article.
-
Molecular signatures of T-cell inhibition in HIV-1 infection.Retrovirology. 2013 Mar 20;10:31. doi: 10.1186/1742-4690-10-31. Retrovirology. 2013. PMID: 23514593 Free PMC article. Review.
-
Aging boosts antiviral CD8+T cell memory through improved engagement of diversified recall response determinants.PLoS Pathog. 2019 Nov 7;15(11):e1008144. doi: 10.1371/journal.ppat.1008144. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31697793 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

