The human T-lymphotropic virus type 1 tax protein inhibits nonsense-mediated mRNA decay by interacting with INT6/EIF3E and UPF1
- PMID: 22553336
- PMCID: PMC3416306
- DOI: 10.1128/JVI.07021-11
The human T-lymphotropic virus type 1 tax protein inhibits nonsense-mediated mRNA decay by interacting with INT6/EIF3E and UPF1
Abstract
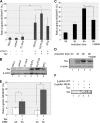
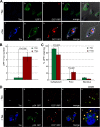
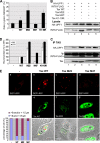
In this report, we analyzed whether the degradation of mRNAs by the nonsense-mediated mRNA decay (NMD) pathway was affected in human T-lymphotropic virus type 1 (HTLV-1)-infected cells. This pathway was indeed strongly inhibited in C91PL, HUT102, and MT2 cells, and such an effect was also observed by the sole expression of the Tax protein in Jurkat and HeLa cells. In line with this activity, Tax binds INT6/EIF3E (here called INT6), which is a subunit of the translation initiation factor eukaryotic initiation factor 3 (eIF3) required for efficient NMD, as well as the NMD core factor upstream frameshift protein 1 (UPF1). It was also observed that Tax expression alters the morphology of processing bodies (P-bodies), the cytoplasmic structures which concentrate RNA degradation factors. The presence of UPF1 in these subcellular compartments was increased by Tax, whereas that of INT6 was decreased. In line with these effects, the level of the phosphorylated form of UPF1 was increased in the presence of Tax. Analysis of several mutants of the viral protein showed that the interaction with INT6 is necessary for NMD inhibition. The alteration of mRNA stability was observed to affect viral transcripts, such as that coding for the HTLV-1 basic leucine zipper factor (HBZ), and also several cellular mRNAs sensitive to the NMD pathway. Our data indicate that the effect of Tax on viral and cellular gene expression is not restricted to transcriptional control but can also involve posttranscriptional regulation.
Figures






References
-
- Abbas S, Erpelinck-Verschueren CA, Goudswaard CS, Lowenberg B, Valk PJ. 2010. Mutant Wilms' tumor 1 (WT1) mRNA with premature termination codons in acute myeloid leukemia (AML) is sensitive to nonsense-mediated RNA decay (NMD). Leukemia 24:660–663 - PubMed
-
- Anczukow O, et al. 2008. Does the nonsense-mediated mRNA decay mechanism prevent the synthesis of truncated BRCA1, CHK2, and p53 proteins? Hum. Mutat. 29:65–73 - PubMed
-
- Asano K, Phan L, Anderson J, Hinnebusch AG. 1998. Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:18573–18585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

