Spindle checkpoint-independent inhibition of mitotic chromosome segregation by Drosophila Mps1
- PMID: 22553353
- PMCID: PMC3374747
- DOI: 10.1091/mbc.E12-02-0117
Spindle checkpoint-independent inhibition of mitotic chromosome segregation by Drosophila Mps1
Abstract
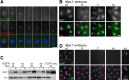
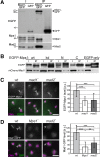
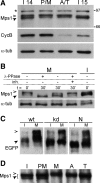
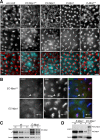
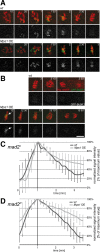
Monopolar spindle 1 (Mps1) is essential for the spindle assembly checkpoint (SAC), which prevents anaphase onset in the presence of misaligned chromosomes. Moreover, Mps1 kinase contributes in a SAC-independent manner to the correction of erroneous initial attachments of chromosomes to the spindle. Our characterization of the Drosophila homologue reveals yet another SAC-independent role. As in yeast, modest overexpression of Drosophila Mps1 is sufficient to delay progression through mitosis during metaphase, even though chromosome congression and metaphase alignment do not appear to be affected. This delay in metaphase depends on the SAC component Mad2. Although Mps1 overexpression in mad2 mutants no longer causes a metaphase delay, it perturbs anaphase. Sister kinetochores barely move apart toward spindle poles. However, kinetochore movements can be restored experimentally by separase-independent resolution of sister chromatid cohesion. We propose therefore that Mps1 inhibits sister chromatid separation in a SAC-independent manner. Moreover, we report unexpected results concerning the requirement of Mps1 dimerization and kinase activity for its kinetochore localization in Drosophila. These findings further expand Mps1's significance for faithful mitotic chromosome segregation and emphasize the importance of its careful regulation.
Figures

References
-
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Buffin E, Emre D, Karess RE. Flies without a spindle checkpoint. Nat Cell Biol. 2007;9:565–572. - PubMed
-
- Buffin E, Lefebvre C, Huang J, Gagou ME, Karess RE. Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr Biol. 2005;15:856–861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

