Models of in vitro spermatogenesis
- PMID: 22553488
- PMCID: PMC3341244
- DOI: 10.4161/spmg.19383
Models of in vitro spermatogenesis
Abstract
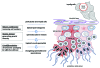
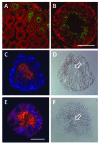
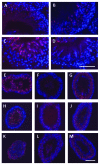
Understanding the mechanisms that lead to the differentiation of male germ cells from their spermatogonial stem cells through meiosis to give rise to mature haploid spermatozoa has been a major quest for many decades. Unlike most other cell types this differentiation process is more or less completely dependent upon the cells being located within the strongly structured niche provided by mature Sertoli cells within an intact seminiferous epithelium. While much new information is currently being obtained through the application and description of relevant gene mutations, there is still a considerable need for in vitro models with which to explore the mechanisms involved. Not only are systems of in vitro spermatogenesis important for understanding the basic science, they have marked pragmatic value in offering ex vivo systems for the artificial maturation of immature germ cells from male infertility patients, as well as providing opportunities for the transgenic manipulation of male germ cells. In this review, we have summarized literature relating to simplistic culturing of germ cells, co-cultures of germ cells with other cell types, especially with Sertoli cells, cultures of seminiferous tubule fragments, and briefly mention the opportunities of xenografting larger testicular pieces. The majority of methods are successful in allowing the differentiation of small steps in the progress of spermatogonia to spermatozoa; few tolerate the chromosomal reduction division through meiosis, and even fewer seem able to complete the complex morphogenesis which results in freely swimming spermatozoa. However, recent progress with complex culture environments, such as 3-d matrices, suggest that possibly success is now not too far away.
Figures

References
-
- Martinovitch PN. Development in vitro of the mammalian gonad. Nature. 1937;139:413. doi: 10.1038/139413a0. - DOI
LinkOut - more resources
Full Text Sources
