Trafficking and proteolytic processing of APP
- PMID: 22553493
- PMCID: PMC3331683
- DOI: 10.1101/cshperspect.a006270
Trafficking and proteolytic processing of APP
Abstract
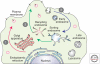
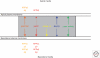
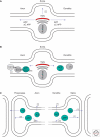
Accumulations of insoluble deposits of amyloid β-peptide are major pathological hallmarks of Alzheimer disease. Amyloid β-peptide is derived by sequential proteolytic processing from a large type I trans-membrane protein, the β-amyloid precursor protein. The proteolytic enzymes involved in its processing are named secretases. β- and γ-secretase liberate by sequential cleavage the neurotoxic amyloid β-peptide, whereas α-secretase prevents its generation by cleaving within the middle of the amyloid domain. In this chapter we describe the cell biological and biochemical characteristics of the three secretase activities involved in the proteolytic processing of the precursor protein. In addition we outline how the precursor protein maturates and traffics through the secretory pathway to reach the subcellular locations where the individual secretases are preferentially active. Furthermore, we illuminate how neuronal activity and mutations which cause familial Alzheimer disease affect amyloid β-peptide generation and therefore disease onset and progression.
Figures

References
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM 2003. ADAMs family members as amyloid precursor protein α-secretases. J Neurosci Res 74: 342–352 - PubMed
-
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK 2005. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis 20: 187–198 - PubMed
-
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T 2001. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem 276: 40353–40361 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
