Vascular development in the zebrafish
- PMID: 22553495
- PMCID: PMC3331685
- DOI: 10.1101/cshperspect.a006684
Vascular development in the zebrafish
Abstract
The zebrafish has emerged as an excellent vertebrate model system for studying blood and lymphatic vascular development. The small size, external and rapid development, and optical transparency of zebrafish embryos are some of the advantages the zebrafish model system offers. Multiple well-established techniques have been developed for imaging and functionally manipulating vascular tissues in zebrafish embryos, expanding on and amplifying these basic advantages and accelerating use of this model system for studying vascular development. In the past decade, studies performed using zebrafish as a model system have provided many novel insights into vascular development. In this article we discuss the amenability of this model system for studying blood vessel development and review contributions made by this system to our understanding of vascular development.
Figures
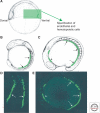
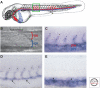


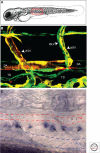
References
-
- Alders M, Hogan BM, Gjini E, Salehi F, Al-Gazali L, Hennekam EA, Holmberg EE, Mannens MM, Mulder MF, Offerhaus GJ, et al. 2009. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet 41: 1272–1274 - PubMed
-
- Bayless KJ, Davis GE 2002. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci 115: 1123–1136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
