Iron metabolism: interactions with normal and disordered erythropoiesis
- PMID: 22553501
- PMCID: PMC3331689
- DOI: 10.1101/cshperspect.a011668
Iron metabolism: interactions with normal and disordered erythropoiesis
Abstract
Hemoglobinopathies and other disorders of erythroid cells are often associated with abnormal iron homeostasis. We review the molecular physiology of intracellular and systemic iron regulation, and the interactions between erythropoiesis and iron homeostasis. Finally, we discuss iron disorders that affect erythropoiesis as well as erythroid disorders that cause iron dysregulation.
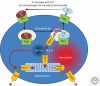
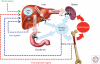
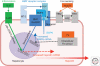
Figures
References
-
- Adamsky K, Weizer O, Amariglio N, Breda L, Harmelin A, Rivella S, Rachmilewitz E, Rechavi G 2004. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol 124: 123–124 - PubMed
-
- Beguin Y, Stray SM, Cazzola M, Huebers HA, Finch CA 1988. Ferrokinetic measurement of erythropoiesis. Acta Haematol 79: 121–126 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
