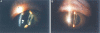F-heparin modified intraocular lenses in Rhesus monkeys
- PMID: 22553538
- PMCID: PMC3340764
- DOI: 10.3980/j.issn.2222-3959.2010.02.11
F-heparin modified intraocular lenses in Rhesus monkeys
Abstract
Aim: In order to improve the biocompatibility of intraocular lenses (IOL), the polymethylmethacrylate (PMMA) IOL was modified with F-heparin.
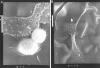
Methods: The PMMA IOL was modified with F ions and heparin by the technique of ion beam combined with low temperature and low pressure plasma. The monkeys (20 eyes) with cataract partly were randomly classified into 2 groups and implanted with PMMA IOL and modified IOL respectively for 180 days. All of the eyes were examined by slit-lamp microscope at postoperative 15, 30, 60, 90, 180 days. The extracted IOL was analyzed with computer image analysis, light microscope (LM) and scanning electron microscope (SEM) at postoperative 180 days.
Results: The early inflammatory reactions postoperatively include anterior chamber exudation and aqueous cell count. The modified IOL group showed less than the non-modified IOL group. The late foreign body cell reaction that adhered to the surface of non-modified IOL was more predominant. The morphologic and pathological changes of posterior capsule opacification (PCO) in monkeys' eyes included fibrosis-type, pearl-type and soemmerring's ring. There was a significant difference between the two groups.
Conclusion: F-heparin modified IOL has good uveal and capsular biocompatibility.
Keywords: F-heparin; Rhesus monkeys; biocompatibility; intraocular lenses; surface modification.
Figures
References
-
- Abela-Formanek C, Amon M, Schild G, Schauersberger J, Heinze G, Kruger A. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28:50–61. - PubMed
-
- Tehrani M, Dick HB, Wolters B, Pakula T, Wolf E. Material properties of various intraocular lenses in an experimental study. Ophthalmologica. 2004;218:57–63. - PubMed
-
- Zetterstrom C, Lundvall A, Olivestedt G. Exfoliation syndrome and heparin surface modified intraocular lenses. Acta Ophthalmologica. 1992;70:91–95. - PubMed
-
- Spängberg M, Kihlström I, Björklund H, Bjurström S, Lydahl E, Larsson R. Improved biocompatibility of intraocular lenses by heparin surface modification: a 12-month implantation study in monkeys. J Cataract Refract Surg. 1990;16:170–177. - PubMed
-
- Amon M. Biocompatibility of Intraocular Lenses. J Cataract Refract Surg. 2001;27:178–179. - PubMed
LinkOut - more resources
Full Text Sources