Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation
- PMID: 22554036
- PMCID: PMC3905677
- DOI: 10.1021/cb300028a
Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation
Abstract
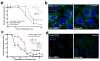
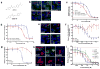
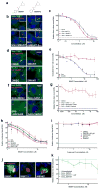
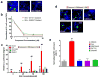
Hedgehog (Hh) signaling promotes tumorigenesis. The accumulation of the membrane protein Smoothened (Smo) within the primary cilium (PC) is a key event in Hh signal transduction, and many pharmacological inhibitors identified to date target Smo's actions. Smo ciliary translocation is inhibited by some pathway antagonists, while others promote ciliary accumulation, an outcome that can lead to a hypersensitive state on renewal of Hh signaling. To identify novel inhibitory compounds acting on the critical mechanistic transition of Smo accumulation, we established a high content screen to directly analyze Smo ciliary translocation. Screening thousands of compounds from annotated libraries of approved drugs and other agents, we identified several new classes of compounds that block Sonic hedgehog-driven Smo localization within the PC. Selective analysis was conducted on two classes of Smo antagonists. One of these, DY131, appears to inhibit Smo signaling through a common binding site shared by previously reported Smo agonists and antagonists. Antagonism by this class of compound is competed by high doses of Smo-binding agonists such as SAG and impaired by a mutation that generates a ligand-independent, oncogenic form of Smo (SmoM2). In contrast, a second antagonist of Smo accumulation within the PC, SMANT, was less sensitive to SAG-mediated competition and inhibited SmoM2 at concentrations similar to those that inhibit wild-type Smo. Our observations identify important differences among Hh antagonists and the potential for development of novel therapeutic approaches against mutant forms of Smo that are resistant to current therapeutic strategies.
Figures

References
-
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. - PubMed
-
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. - PubMed
-
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1 (+/−) p53 (−/−) mice. Cancer Cell. 2004;6:229–240. - PubMed
-
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. - PubMed
-
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nature Reviews Drug Discovery. 2006;5:1026–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

