Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia
- PMID: 22554203
- PMCID: PMC3464686
- DOI: 10.1186/1475-2875-11-153
Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia
Abstract
Background: A practical and simple regimen for all malaria species is needed towards malaria elimination in Indonesia. It is worth to compare the efficacy and safety of a single dose of artemisinin-naphthoquine (AN) with a three-day regimen of dihydroartemisinin-piperaquine (DHP), the existing programme drug, in adults with uncomplicated symptomatic malaria.
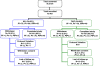
Methods: This is a phase III, randomized, open label using sealed envelopes, multi-centre, comparative study between a single dose of AN and a three-day dose of DHP in Jayapura and Maumere. The modified WHO inclusion and exclusion criteria for efficacy study were used in this trial. A total of 401 eligible adult malaria subjects were hospitalized for three days and randomly treated with AN four tablets single dose on day 0 or DHP three to four tablets single daily dose for three days, and followed for 42 days for physical examination, thick and thin smears microscopy, and other necessary tests. The efficacy of drug was assessed by polymerase chain reaction (PCR) uncorrected and corrected.
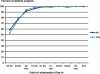
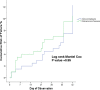
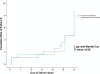
Results: There were 153 Plasmodium falciparum, 158 Plasmodium vivax and 90 P. falciparum/P. vivax malaria. Mean of fever clearance times were similar, 13.0 ± 10.3 hours in AN and 11.3 ± 7.3 hours in DHP groups. The mean of parasite clearance times were longer in AN compared with DHP (28.0 ± 11.7 hours vs 25.5 ± 12.2 hours, p = 0.04). There were only 12 PCR-corrected P. falciparum late treatment failures: seven in AN and five in DHP groups. The PCR uncorrected and corrected on day -42 of adequate clinical and parasitological responses for treatment of any malaria were 93.7% (95% Cl: 90.3-97.2) and 96.3% (95% Cl: 93.6-99.0) in AN, 96.3% (95% Cl: 93.5-99.0) and 97.3% (95% Cl: 95.0-99.6) in DHP groups. Few and mild adverse events were reported. All the abnormal haematology and blood chemistry values had no clinical abnormality.
Conclusion: AN and DHP are confirmed very effective, safe and tolerate for treatment of any malaria. Both drugs are promising for multiple first-line therapy policies in Indonesia.
Figures


References
-
- World Health Organization. World Malaria Report 2008. WHO/HTM/GMP/2008.1. Geneva: WHO; 2008.
-
- World Health Organization. Antimalarial drug combination therapy. Geneva: WHO; 2001.
-
- Price RN, Nosten F, Luxemburger C, Ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, White NJ. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. - PubMed
-
- Nosten F, van Vugt M, Price RN, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. Effects of artesunate-mefloquine combination on incidence ofPlasmodium falciparummalaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. - PubMed
-
- Tjitra E, Gunawan S, Laihad F, Marwoto H, Sulaksono S, Arjoso S, Richie TL, Manurung N. Evaluation of antimalaria drugs in Indonesia, 1981–1995. Bull Hlth Studies. 1997;25:27–58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

