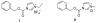Efficient synthesis of exo-N-carbamoyl nucleosides: application to the synthesis of phosphoramidate prodrugs
- PMID: 22554490
- PMCID: PMC3432299
- DOI: 10.1021/ol300777p
Efficient synthesis of exo-N-carbamoyl nucleosides: application to the synthesis of phosphoramidate prodrugs
Abstract
An efficient protection protocol for the 6-exo-amino group of purine nucleosides with various chloroformates was developed utilizing N-methylimidazole (NMI). The reaction of an exo-N(6)-group of adenosine analogue 1 with alkyl/and aryl chloroformates under optimized conditions provided the N(6)-carbamoyl adenosines (2a-j) in good to excellent yields. The reaction of N(6)-Cbz-protected nucleosides (5a-c) with phenyl phosphoryl chloride (7) using t-BuMgCl followed by catalytic hydrogenation afforded the corresponding phosphoramidate pronucleotides (8a-c) in excellent yield.
Figures
References
-
- Wuts PGM, Greene TW. Green’s Protective Groups in Organic Synthesis. 4th edition John Wiley and Sons, Inc.; Hoboken, NJ: 2007.
- Kocienski PJ. Protecting Groups; Geoug Thieme Verlag Stuttgart. New York: 1994.
-
- Samano V, Miles RW, Robins MJ. J. Am. Chem. Soc. 1994;116:9331–9332.
- Miles RW, Samano V, Robins MJ. J. Am. Chem. Soc. 1995;117:5951–5957.
- Cui Z, Zhang L, Zhang B. Tetrahedron Lett. 2001;42:561–563.
- Nguyen-Trung NQ, Terenzi S, Scherer G, Strazewski P. Org. Lett. 2003;5:2603–2606. - PubMed
- Nowak I, Robins MJ. Org. Lett. 2003;5:3345–3348. - PubMed
- Zhong M, Nowak I, Robins MJ. J. Org. Chem. 2006;71:7773–7779. - PubMed
- Derudas M, Carta D, Brancale A, Vanpouille C, Lisco A, Margolis L, Balzarini J, McGuigan C. J. Med. Chem. 2009;52:5520–5530. - PMC - PubMed
- Arico JW, Calhoun AK, Salandria KJ, McLaughlin LW. Org. Lett. 2010;12:120–122. - PubMed
-
-
For reviews: Bobeck DR, Coats SJ, Schinazi RF. Antivir. Ther. 2010;15:935–950. Erion MD, Hecker SJ. J. Med. Chem. 2008;51:2328–2345. Schultz C. Bioorg. Med. Chem. 2003;11:885–898. Mehellou Y, Balzarini J, McGuigan C. Chem. Med. Chem. 2009;4:1779–1791. Sofia MJ. Antivir. Chem. Chemother. 2011;22:23–49.
-
-
- Hotoda H, Saito R, Sekine M, Hata T. Tetrahedron. 1990;46:1181–1190.
- Strasser M, Ugi I. Acta Chem. Scand. 1993;47:125–130.
-
- Dey S, Garner PJ. Org. Chem. 2000;65:7697–7699. - PubMed
- Bazzanini R, Gouy M:H, Peyrottes S, Gosselin G, Perigaud C, Manfrendini S. Nucleosides, Nucleotides & Nucleic acids. 2005;24:1635–1649. - PubMed
- Wojciechowski F, Hudson RHE. J. Org. Chem. 2008;73:3807–3816. - PubMed
- Wang R:W, Gold B. Org. Lett. 2009;11:2465–2468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources