Ezrin functionality and hypothermic preservation injury in LLC-PK1 cells
- PMID: 22554620
- PMCID: PMC3367074
- DOI: 10.1016/j.cryobiol.2012.04.003
Ezrin functionality and hypothermic preservation injury in LLC-PK1 cells
Abstract
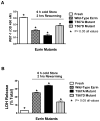
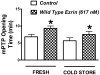
Renal epithelial cells from donor kidneys are susceptible to hypothermic preservation injury, which is attenuated when they over express the cytoskeletal linker protein ezrin. This study was designed to characterize the mechanisms of this protection. Renal epithelial cell lines were created from LLC-PK1 cells, which expressed mutant forms of ezrin with site directed alterations in membrane binding functionality. The study used cells expressing wild type ezrin, T567A, and T567D ezrin point mutants. The A and D mutants have constitutively inactive and active membrane binding conformations, respectively. Cells were cold stored (4 °C) for 6-24 h and reperfused for 1h to simulate transplant preservation injury. Preservation injury was assessed by mitochondrial activity (WST-1) and LDH release. Cells expressing the active ezrin mutant (T567D) showed significantly less preservation injury compared to wild type or the inactive mutant (T567A), while ezrin-specific siRNA knockdown and the inactive mutant potentiated preservation injury. Ezrin was extracted and identified from purified mitochondria. Furthermore, isolated mitochondria specifically bound anti-ezrin antibodies, which were reversed with the addition of exogenous recombinant ezrin. Recombinant wild type ezrin significantly reduced the sensitivity of the mitochondrial permeability transition pore (mPTP) to calcium, suggesting ezrin may modify mitochondrial function. In conclusion, the cytoskeletal linker protein ezrin plays a significant role in hypothermic preservation injury in renal epithelia. The mechanisms appear dependent on the molecule's open configuration (traditional linker functionality) and possibly a novel mitochondrial specific role, which may include modulation of mPTP function or calcium sensitivity.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Ezrin and its emerging role in tumor progression in systemic malignancies.Cryobiology. 2013 Apr;66(2):93. doi: 10.1016/j.cryobiol.2012.11.009. Epub 2012 Dec 21. Cryobiology. 2013. PMID: 23261414 No abstract available.
-
Ezrin and its emerging role in tumor progression: response.Cryobiology. 2013 Apr;66(2):94. doi: 10.1016/j.cryobiol.2012.11.010. Epub 2012 Dec 27. Cryobiology. 2013. PMID: 23274401 Free PMC article. No abstract available.
References
-
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. - PubMed
-
- Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, Kashgarian M, van Why SK. Ischemic conditioning prevents Na, K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002;51:722–727. - PubMed
-
- Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

