SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons
- PMID: 22554781
- PMCID: PMC3383402
- DOI: 10.1016/j.neuroscience.2012.04.053
SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons
Abstract
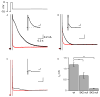
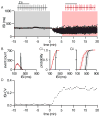
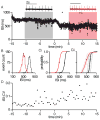
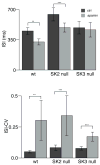
The firing properties of dopamine (DA) neurons in the substantia nigra (SN) pars compacta are strongly influenced by the activity of apamin-sensitive small conductance Ca(2+)-activated K(+) (SK) channels. Of the three SK channel genes expressed in central neurons, only SK3 expression has been identified in DA neurons. The present findings show that SK2 was also expressed in DA neurons. Immuno-electron microscopy (iEM) showed that SK2 was primarily expressed in the distal dendrites, while SK3 was heavily expressed in the soma and, to a lesser extent, throughout the dendritic arbor. Electrophysiological recordings of the effects of the SK channel blocker apamin on DA neurons from wild type and SK(-/-) mice show that SK2-containing channels contributed to the precision of action potential (AP) timing, while SK3-containing channels influenced AP frequency. The expression of SK2 in DA neurons may endow distinct signaling and subcellular localization to SK2-containing channels.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. - PubMed
-
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. - PubMed
-
- Chergui K, Suaud-Chagny MF, Gonon FG. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

