Mating-type genes and MAT switching in Saccharomyces cerevisiae
- PMID: 22555442
- PMCID: PMC3338269
- DOI: 10.1534/genetics.111.134577
Mating-type genes and MAT switching in Saccharomyces cerevisiae
Abstract
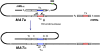
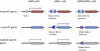
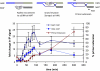
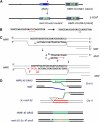
Mating type in Saccharomyces cerevisiae is determined by two nonhomologous alleles, MATa and MATα. These sequences encode regulators of the two different haploid mating types and of the diploids formed by their conjugation. Analysis of the MATa1, MATα1, and MATα2 alleles provided one of the earliest models of cell-type specification by transcriptional activators and repressors. Remarkably, homothallic yeast cells can switch their mating type as often as every generation by a highly choreographed, site-specific homologous recombination event that replaces one MAT allele with different DNA sequences encoding the opposite MAT allele. This replacement process involves the participation of two intact but unexpressed copies of mating-type information at the heterochromatic loci, HMLα and HMRa, which are located at opposite ends of the same chromosome-encoding MAT. The study of MAT switching has yielded important insights into the control of cell lineage, the silencing of gene expression, the formation of heterochromatin, and the regulation of accessibility of the donor sequences. Real-time analysis of MAT switching has provided the most detailed description of the molecular events that occur during the homologous recombinational repair of a programmed double-strand chromosome break.
Figures







References
-
- Abraham J., Nasmyth K. A., Strathern J. N., Klar A. J., Hicks J. B., 1984. Regulation of mating-type information in yeast. Negative control requiring sequences both 5′ and 3′ to the regulated region. J. Mol. Biol. 176: 307–331. - PubMed
-
- Amati B. B., Gasser S. M., 1988. Chromosomal ARS and CEN elements bind specifically to the yeast nuclear scaffold. Cell 54: 967–978. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

