Knowledge integration at the center of genomic medicine
- PMID: 22555656
- PMCID: PMC4681509
- DOI: 10.1038/gim.2012.43
Knowledge integration at the center of genomic medicine
Abstract
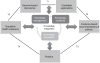
Three articles in this issue of Genetics in Medicine describe examples of "knowledge integration," involving methods for generating and synthesizing rapidly emerging information on health-related genomic technologies and engaging stakeholders around the evidence. Knowledge integration, the central process in translating genomic research, involves three closely related, iterative components: knowledge management, knowledge synthesis, and knowledge translation. Knowledge management is the ongoing process of obtaining, organizing, and displaying evolving evidence. For example, horizon scanning and "infoveillance" use emerging technologies to scan databases, registries, publications, and cyberspace for information on genomic applications. Knowledge synthesis is the process of conducting systematic reviews using a priori rules of evidence. For example, methods including meta-analysis, decision analysis, and modeling can be used to combine information from basic, clinical, and population research. Knowledge translation refers to stakeholder engagement and brokering to influence policy, guidelines and recommendations, as well as the research agenda to close knowledge gaps. The ultrarapid production of information requires adequate public and private resources for knowledge integration to support the evidence-based development of genomic medicine.
Conflict of interest statement
DISCLOSURE
The authors declare no conflict of interest.
Figures
References
-
- Green ED, Guyer MS, National Human Genome Research Institute Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. - PubMed
-
- McDermott U, Downing JR, Stratton MR. Genomics and the continuum of cancer care. N Engl J Med. 2011;364:340–350. - PubMed
-
- Institute of Medicine Roundtable on Translating Genome-based Research for Health. Generating Evidence for Genomic Diagnostic Test Development: A Workshop Summary. National Academies Press; Washington, DC: 2011. - PubMed
-
- National Cancer Institute. Comparative effectiveness research in genomics and personalized medicine. http://cancercontrol.cancer.gov/od/phg/research.asp?type=CER. Accessed 1 February 2011.
-
- Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151:203–205. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources