Synthesis and structure-activity relationship studies of HIV-1 virion infectivity factor (Vif) inhibitors that block viral replication
- PMID: 22555953
- PMCID: PMC3517065
- DOI: 10.1002/cmdc.201200079
Synthesis and structure-activity relationship studies of HIV-1 virion infectivity factor (Vif) inhibitors that block viral replication
Abstract
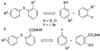
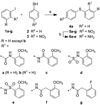
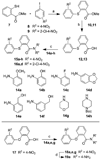
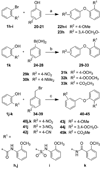
The human immunodeficiency virus 1 (HIV-1) virion infectivity factor (Vif) protein, essential for in vivo viral replication, protects the virus from innate antiviral cellular factor apolipoprotein B mRNA-editing, enzyme-catalytic, polypeptide-like 3G (APOBEC3G; A3G) and is an attractive target for the development of novel antiviral therapeutics. We have evaluated the structure-activity relationships of N-(2-methoxyphenyl)-2-((4-nitrophenyl)thio)benzamide (RN-18), a small molecule recently identified as an inhibitor of Vif function that blocks viral replication only in nonpermissive cells expressing A3G, by inhibiting Vif-A3G interactions. Microwave-assisted cross-coupling reactions were developed to prepare a series of RN18 analogues with diverse linkages and substitutions on the phenyl rings. A dual cell-based assay system was used to assess antiviral activity against wild-type HIV-1 in both nonpermissive (H9) and permissive (MT4) cells that also allowed evaluation of specificity. In general, variations of phenyl substitutions were detrimental to antiviral potency and specificity, but isosteric replacements of amide and ether linkages were relatively well tolerated. These structure-activity relationship data define structural requirements for Vif-specific activity, identify new compounds with improved antiviral potency and specificity, and provide leads for further exploration to develop new antiviral therapeutics.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Joint United Nations Program on HIV/AIDS (UNAIDS) UNAIDS Report on the Global AIDS Epidemic 2010, UNAIDS. Geneva, Switzerland: 2010.
-
- Mehellou Y, De Clercq E. J. Med. Chem. 2010;53:521–538. - PubMed
-
- Thompson MA, Aberg JA, Cahn P, Montaner JSG, Rizzardini G, Telenti A, Gatell JM, Günthard HF, Hammer SM, Hirsch MS, Jacobsen DM, Reiss P, Richman DD, Volberding PA, Yeni P, Schooley RT. JAMA. 2010;304:321–333. - PubMed
-
- Volberding PA, Deeks SG. The Lancet. 2010;376:49–62. - PubMed
-
- Fisher AG, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo RC, Wong-Staal F. Science. 1987;237:888–893. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

