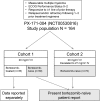
An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma
- PMID: 22555973
- PMCID: PMC4123327
- DOI: 10.1182/blood-2012-03-414359
An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma
Abstract
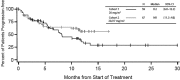
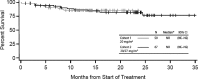
Carfilzomib is a selective proteasome inhibitor that binds irreversibly to its target. In phase 1 studies, carfilzomib elicited promising responses and an acceptable toxicity profile in patients with relapsed and/or refractory multiple myeloma (R/R MM). In the present phase 2, multicenter, open-label study, 129 bortezomib-naive patients with R/R MM (median of 2 prior therapies) were separated into Cohort 1, scheduled to receive intravenous carfilzomib 20 mg/m(2) for all treatment cycles, and Cohort 2, scheduled to receive 20 mg/m(2) for cycle 1 and then 27 mg/m(2) for all subsequent cycles. The primary end point was an overall response rate (≥ partial response) of 42.4% in Cohort 1 and 52.2% in Cohort 2. The clinical benefit response (overall response rate + minimal response) was 59.3% and 64.2% in Cohorts 1 and 2, respectively. Median duration of response was 13.1 months and not reached, and median time to progression was 8.3 months and not reached, respectively. The most common treatment-emergent adverse events were fatigue (62.0%) and nausea (48.8%). Single-agent carfilzomib elicited a low incidence of peripheral neuropathy-17.1% overall (1 grade 3; no grade 4)-in these pretreated bortezomib-naive patients. The results of the present study support the use of carfilzomib in R/R MM patients. This trial is registered at www.clinicaltrials.gov as NCT00530816.
Figures
References
-
- American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012.
-
- Laubach J, Mitsiades C, Mahindra A, et al. Management of relapsed and relapsed/refractory multiple myeloma. J Natl Compr Canc Netw. 2011;9(10):1209–1216. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
-
- Dorsey BD, Iqbal M, Chatterjee S, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51(4):1068–1072. - PubMed