The role of cyclooxygenase-2 in mechanical ventilation-induced lung injury
- PMID: 22556158
- PMCID: PMC3488687
- DOI: 10.1165/rcmb.2011-0005OC
The role of cyclooxygenase-2 in mechanical ventilation-induced lung injury
Abstract
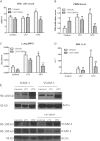
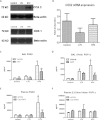
Mechanical ventilation is necessary for patients with acute respiratory failure, but can cause or propagate lung injury. We previously identified cyclooxygenase-2 as a candidate gene in mechanical ventilation-induced lung injury. Our objective was to determine the role of cyclooxygenase-2 in mechanical ventilation-induced lung injury and the effects of cyclooxygenase-2 inhibition on lung inflammation and barrier disruption. Mice were mechanically ventilated at low and high tidal volumes, in the presence or absence of pharmacologic cyclooxygenase-2-specific inhibition with 3-(4-methylsulphonylphenyl)-4-phenyl-5-trifluoromethylisoxazole (CAY10404). Lung injury was assessed using markers of alveolar-capillary leakage and lung inflammation. Cyclooxygenase-2 expression and activity were measured by Western blotting, real-time PCR, and lung/plasma prostanoid analysis, and tissue sections were analyzed for cyclooxygenase-2 staining by immunohistochemistry. High tidal volume ventilation induced lung injury, significantly increasing both lung leakage and lung inflammation relative to control and low tidal volume ventilation. High tidal volume mechanical ventilation significantly induced cyclooxygenase-2 expression and activity, both in the lungs and systemically, compared with control mice and low tidal volume mice. The immunohistochemical analysis of lung sections localized cyclooxygenase-2 expression to monocytes and macrophages in the alveoli. The pharmacologic inhibition of cyclooxygenase-2 with CAY10404 significantly decreased cyclooxygenase activity and attenuated lung injury in mice ventilated at high tidal volume, attenuating barrier disruption, tissue inflammation, and inflammatory cell signaling. This study demonstrates the induction of cyclooxygenase-2 by mechanical ventilation, and suggests that the therapeutic inhibition of cyclooxygenase-2 may attenuate ventilator-induced acute lung injury.
Figures


References
-
- Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 - PubMed
-
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54–61 - PubMed
-
- Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome: III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med 2000;26:1215–1227 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

