Uterotonic drug quality: an assessment of the potency of injectable uterotonic drugs purchased by simulated clients in three districts in Ghana
- PMID: 22556159
- PMCID: PMC3346944
- DOI: 10.1136/bmjopen-2011-000431
Uterotonic drug quality: an assessment of the potency of injectable uterotonic drugs purchased by simulated clients in three districts in Ghana
Abstract
Objectives: Given use of uterotonics for postpartum haemorrhage and other obstetric indications, the importance of potent uterotonics is indisputable. This study evaluated access to and potency of injectable uterotonics in Ghana.
Design: Study design involved research assistants simulating clients to purchase oxytocin and ergometrine from different sources. Drug potency was measured via chemical assay by the Ghana Food and Drugs Board.
Setting: The study was conducted in three contrasting districts in Ghana.
Outcome measure: The per cent of active pharmaceutical ingredient was measured to assess the quality of oxytocin and ergometrine.
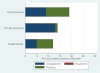
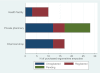
Results: 69 formal points of sale were visited, from which 55 ergometrine ampoules and 46 oxytocin ampoules were purchased. None of the ergometrine ampoules were within British Pharmacopoeia specification for active ingredient, none were expired and one showed 0% active ingredient, suggestive of a counterfeit drug. Among oxytocin ampoules purchased, only 11 (26%) were within British Pharmacopoeia specification for active ingredient and two (4%) were expired. The median percentages of active ingredients were 64% and 50% for oxytocin and ergometrine, respectively.
Conclusions: The quality of injectable uterotonics in three contrasting districts in Ghana is a serious problem. Restrictions regarding the sale of unregistered drugs, and of registered drugs from unlicensed shops, are inadequately enforced. These problems likely exist elsewhere but are not assessed, as postmarketing drug quality surveillance is generally restricted to well-funded disease-specific programmes relying on antiretroviral, antimalarial and antibiotic drugs. Maternal health programmes must adopt and fund the same approach to drug quality as is standard in programmes addressing infectious disease.
Conflict of interest statement
Figures
References
-
- WHO WHO Model List of Essential Medicines; 16th List (Updated). Geneva, 2010. http://www.who.int/medicines/publications/essentialmedicines/en/index.html
-
- WHO WHO Statement Regarding the Use of Misoprostol for Postpartum Haemorrhage Prevention and Treatment. Geneva: Department of Reproductive Health and Research, 2009
-
- WHO WHO List of Priority Medicines for Mothers and Children. Department of Maternal, Newborn, Child and Adolescent Health, 2011
-
- Stability of Injectable Oxytocics in Tropical Climates; Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. Geneva: Action Program on Essential Drugs, World Health Organisation, 1993
-
- de Groot AN, Hekster YA, Vree TB, et al. Ergometrine and methylergometrine tablets are not stable under simulated tropical conditions. J Clin Pharm Ther 1995;20:109–13 - PubMed
LinkOut - more resources
Full Text Sources