Effects of an online personal health record on medication accuracy and safety: a cluster-randomized trial
- PMID: 22556186
- PMCID: PMC3422826
- DOI: 10.1136/amiajnl-2011-000723
Effects of an online personal health record on medication accuracy and safety: a cluster-randomized trial
Abstract
Objective: To determine the effects of a personal health record (PHR)-linked medications module on medication accuracy and safety.
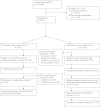
Design: From September 2005 to March 2007, we conducted an on-treatment sub-study within a cluster-randomized trial involving 11 primary care practices that used the same PHR. Intervention practices received access to a medications module prompting patients to review their documented medications and identify discrepancies, generating 'eJournals' that enabled rapid updating of medication lists during subsequent clinical visits.
Measurements: A sample of 267 patients who submitted medications eJournals was contacted by phone 3 weeks after an eligible visit and compared with a matched sample of 274 patients in control practices that received a different PHR-linked intervention. Two blinded physician adjudicators determined unexplained discrepancies between documented and patient-reported medication regimens. The primary outcome was proportion of medications per patient with unexplained discrepancies.
Results: Among 121,046 patients in eligible practices, 3979 participated in the main trial and 541 participated in the sub-study. The proportion of medications per patient with unexplained discrepancies was 42% in the intervention arm and 51% in the control arm (adjusted OR 0.71, 95% CI 0.54 to 0.94, p=0.01). The number of unexplained discrepancies per patient with potential for severe harm was 0.03 in the intervention arm and 0.08 in the control arm (adjusted RR 0.31, 95% CI 0.10 to 0.92, p=0.04).
Conclusions: When used, concordance between documented and patient-reported medication regimens and reduction in potentially harmful medication discrepancies can be improved with a PHR medication review tool linked to the provider's medical record.
Trial registration number: This study was registered at ClinicalTrials.gov (NCT00251875).
Conflict of interest statement
Figures
References
-
- Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med 1995;155:1949–56 - PubMed
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29–34 - PubMed
-
- Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003;348:1556–64 - PubMed
-
- Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71 - PubMed
-
- Insull W. The problem of compliance to cholesterol altering therapy. J Intern Med 1997;241:317–25 - PubMed

