Loss of fibroblast HIF-1α accelerates tumorigenesis
- PMID: 22556263
- PMCID: PMC4089958
- DOI: 10.1158/0008-5472.CAN-12-0534
Loss of fibroblast HIF-1α accelerates tumorigenesis
Abstract
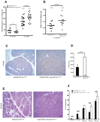
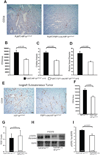
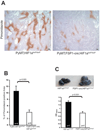
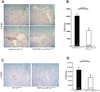
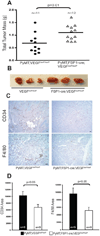
Solid tumors consist of malignant cells and associated stromal components, including fibroblastic cells that contribute to tumor growth and progression. Although tumor fibrosis and aberrant vascularization contribute to the hypoxia often found in advanced tumors, the contribution of hypoxic signaling within tumor-associated fibroblasts to tumorigenesis remains unknown. In this study, we used a fibroblast-specific promoter to create mice in which key hypoxia regulatory genes, including VHL, HIF-1α, HIF-2α, and VEGF-A, were knocked out specifically in tumor stromal fibroblasts. We found that loss of HIF-1α and its target gene VEGF-A accelerated tumor growth in murine model of mammary cancer. HIF-1α and VEGF-A loss also led to a reduction in vascular density and myeloid cell infiltration, which correlated with improved tumor perfusion. Together, our findings indicate that the fibroblast HIF-1α response is a critical component of tumor vascularization.
©2012 AACR.
Conflict of interest statement
The authors declare no conflict of interest
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

