Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells
- PMID: 22556268
- PMCID: PMC3384206
- DOI: 10.1073/pnas.1200781109
Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells
Abstract
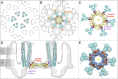
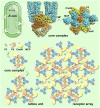
The chemoreceptors of Escherichia coli localize to the cell poles and form a highly ordered array in concert with the CheA kinase and the CheW coupling factor. However, a high-resolution structure of the array has been lacking, and the molecular basis of array assembly has thus remained elusive. Here, we use cryoelectron tomography of flagellated E. coli minicells to derive a 3D map of the intact array. Docking of high-resolution structures into the 3D map provides a model of the core signaling complex, in which a CheA/CheW dimer bridges two adjacent receptor trimers via multiple hydrophobic interactions. A further, hitherto unknown, hydrophobic interaction between CheW and the homologous P5 domain of CheA in an adjacent core complex connects the complexes into an extended array. This architecture provides a structural basis for array formation and could explain the high sensitivity and cooperativity of chemotaxis signaling in E. coli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

