Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis
- PMID: 22556332
- PMCID: PMC3385654
- DOI: 10.1161/ATVBAHA.112.250142
Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis
Abstract
Objective: Elevated serum free fatty acid levels are associated with an increased risk of cardiovascular disease and type 2 diabetes mellitus. Macrophages are recruited to atherosclerotic plaques and metabolic tissues during obesity and accumulate lipids, including free fatty acids. We investigated the molecular consequences of intracellular saturated free fatty acid accumulation in macrophages.
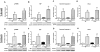
Methods and results: Previously, we demonstrated that cotreatment of mouse peritoneal macrophages (MPMs) with stearic acid and triacsin C (an inhibitor of long-chain acyl coenzyme A synthetases) results in intracellular free fatty acid accumulation and apoptosis. Here, we used Western blotting analysis, real-time reverse transcription polymerase chain reaction, and terminal deoxynucleotidyl transferase dUTP nick-end labeling staining to assess endoplasmic reticulum (ER) stress, inflammation, and apoptosis in MPMs. Intracellular stearic acid accumulation induces Toll-like receptor 4/2-independent inflammation that results in ER stress-mediated apoptosis of MPMs. Polarization of MPMs to a proinflammatory M1 phenotype increases their susceptibility to inflammation and ER stress, but not apoptosis, in response to cotreatment with stearic acid and triacsin C.
Conclusions: Intracellular accumulation of stearic acid in MPMs activates inflammatory signaling, leading to ER stress-mediated apoptosis. M1 macrophages are more prone to stearic acid-induced inflammation and ER stress. These same pathways may be activated in macrophages residing in atherosclerotic plaques and metabolic tissues during conditions of obesity and hyperlipidemia.
Conflict of interest statement
Figures

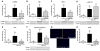

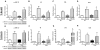

References
-
- Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH. Fasting plasma free fatty acids and risk of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. - PubMed
-
- Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R01 HL089466/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- HL089466/HL/NHLBI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- T32GM008554/GM/NIGMS NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- T32 HL007411/HL/NHLBI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 GM008554/GM/NIGMS NIH HHS/United States
- T32 HL007411-30/HL/NHLBI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

