Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells
- PMID: 22556413
- PMCID: PMC3375534
- DOI: 10.1074/jbc.M111.319129
Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells
Abstract
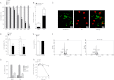
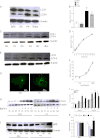
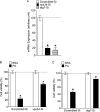
Accumulation of palmitic acid (PA) in cells from nonadipose tissues is known to induce lipotoxicity resulting in cellular dysfunction and death. The exact molecular pathways of PA-induced cell death are still mysterious. Here, we show that PA triggers autophagy, which did not counteract but in contrast promoted endothelial cell death. The PA-induced cell death was predominantly necrotic as indicated by annexin V and propidium iodide (PI) staining, absence of caspase activity, low levels of DNA hypoploidy, and an early ATP depletion. In addition PA induced a strong elevation of mRNA levels of ubiquitin carboxyl-terminal hydrolase (CYLD), a known mediator of necroptosis. Moreover, siRNA-mediated knockdown of CYLD significantly antagonized PA-induced necrosis of endothelial cells. In contrast, inhibition and knockdown of receptor interacting protein kinase 1 (RIPK1) had no effect on PA-induced necrosis, indicating the induction of a CYLD-dependent but RIPK1-independent cell death pathway. PA was recognized as a strong and early inducer of autophagy. The inhibition of autophagy by both pharmacological inhibitors and genetic knockdown of the autophagy-specific genes, vacuolar protein sorting 34 (VPS34), and autophagy-related protein 7 (ATG7), could rescue the PA-induced death of endothelial cells. Moreover, the initiation of autophagy and cell death by PA was reduced in endothelial cells loaded with the Ca(2+) chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid-(acetoxymethyl) ester (BAPTA-AM), indicating that Ca(2+) triggers the fatal signaling of PA. In summary, we introduce an unexpected mechanism of lipotoxicity in endothelial cells and provide several novel strategies to counteract the lipotoxic signaling of PA.
Figures



References
-
- Unger R., Zhou Y. (2001) Lipotoxicity of beta cells in obesity and in other causes of fatty acid spillover. Diabetes 1, S118–S121 - PubMed
-
- Borradaile N. M., Schaffer J. E. (2005) Lipotoxicity in the heart. Curr. Hypertens. Rep. 7, 412–417 - PubMed
-
- Pfaffenbach K. T., Gentile C. L., Nivala A. M., Wang D., Wei Y., Pagliassotti M. J. (2010) Linking endoplasmic reticulum stress to cell death in hepatocytes. Roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am. J. Physiol. Endocrinol. Metab. 298, E1027–E1035 - PMC - PubMed
-
- Mayer C. M., Belsham D. D. (2010) Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons. Rescue of resistance and apoptosis through adenosine 5′-monophosphate-activated protein kinase activation. Endocrinology. 151, 576–585 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

