Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells
- PMID: 22556418
- PMCID: PMC3375550
- DOI: 10.1074/jbc.M112.357202
Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells
Abstract
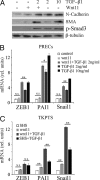
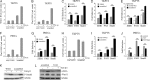
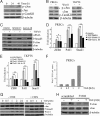
Transforming growth factor β1 (TGF-β) promotes renal interstitial fibrosis in vivo and the expression of mesenchymal genes in vitro; however, most of its direct targets in epithelial cells are still elusive. In a screen for genes directly activated by TGF-β, we found that components of the Wnt signaling pathway, especially Wnt11, were targets of activation by TGF-β and Smad3 in primary renal epithelial cells. In gain and loss of function experiments, Wnt11 mediated the actions of TGF-β through enhanced activation of mesenchymal marker genes, such as Zeb1, Snail1, Pai1, and αSMA, without affecting Smad3 phosphorylation. Inhibition of Wnt11 by receptor knockdown or treatment with Wnt inhibitors limited the effects of TGF-β on gene expression. We found no evidence that Wnt11 activated the canonical Wnt signaling pathway in renal epithelial cells; rather, the function of Wnt11 was mediated by the c-Jun N-terminal kinase (JNK) pathway. Consistent with the in vitro results, all the TGF-β, Wnt11, and JNK targets were activated in a unilateral ureteral obstruction (UUO) model of renal fibrosis in vivo. Our findings demonstrated cooperativity among the TGF-β, Wnt11, and JNK signaling pathways and suggest new targets for anti-fibrotic therapy in renal tissue.
Figures




References
-
- Böttinger E. P., Bitzer M. (2002) TGF-β signaling in renal disease. J. Am. Soc. Nephrol. 13, 2600–2610 - PubMed
-
- Zeisberg E. M., Tarnavski O., Zeisberg M., Dorfman A. L., McMullen J. R., Gustafsson E., Chandraker A., Yuan X., Pu W. T., Roberts A. B., Neilson E. G., Sayegh M. H., Izumo S., Kalluri R. (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 - PubMed
-
- Kopp J. B., Factor V. M., Mozes M., Nagy P., Sanderson N., Böttinger E. P., Klotman P. E., Thorgeirsson S. S. (1996) Transgenic mice with increased plasma levels of TGF-β 1 develop progressive renal disease. Lab. Invest. 74, 991–1003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

