Critical role of filamin-binding LIM protein 1 (FBLP-1)/migfilin in regulation of bone remodeling
- PMID: 22556421
- PMCID: PMC3375566
- DOI: 10.1074/jbc.M111.331249
Critical role of filamin-binding LIM protein 1 (FBLP-1)/migfilin in regulation of bone remodeling
Abstract
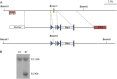
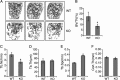
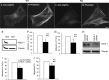
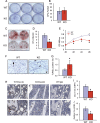
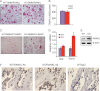
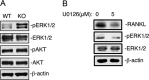
Bone remodeling is a complex process that must be precisely controlled to maintain a healthy life. We show here that filamin-binding LIM protein 1 (FBLP-1, also known as migfilin), a kindlin- and filamin-binding focal adhesion protein, is essential for proper control of bone remodeling. Genetic inactivation of FBLIM1 (the gene encoding FBLP-1) in mice resulted in a severe osteopenic phenotype. Primary FBLP-1 null bone marrow stromal cells (BMSCs) exhibited significantly reduced extracellular matrix adhesion and migration compared with wild type BMSCs. Loss of FBLP-1 significantly impaired the growth and survival of BMSCs in vitro and decreased the number of osteoblast (OB) progenitors in bone marrow and OB differentiation in vivo. Furthermore, the loss of FBLP-1 caused a dramatic increase of osteoclast (OCL) differentiation in vivo. The level of receptor activator of nuclear factor κB ligand (RANKL), a key regulator of OCL differentiation, was markedly increased in FBLP-1 null BMSCs. The capacity of FBLP-1 null bone marrow monocytes (BMMs) to differentiate into multinucleated OCLs in response to exogenously supplied RANKL, however, was not different from that of WT BMMs. Finally, we show that a loss of FBLP-1 promotes activating phosphorylation of ERK1/2. Inhibition of ERK1/2 activation substantially suppressed the increase of RANKL induced by the loss of FBLP-1. Our results identify FBLP-1 as a key regulator of bone homeostasis and suggest that FBLP-1 functions in this process through modulating both the intrinsic properties of OB/BMSCs (i.e., BMSC-extracellular matrix adhesion and migration, cell growth, survival, and differentiation) and the communication between OB/BMSCs and BMMs (i.e., RANKL expression) that controls osteoclastogenesis.
Figures

References
-
- Ash P., Loutit J. F., Townsend K. M. (1980) Osteoclasts derived from haematopoietic stem cells. Nature 283, 669–670 - PubMed
-
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 - PubMed
-
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 - PMC - PubMed
-
- Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T. M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W. J. (1997) Osteoprotegerin. A novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

