Crystal structure of elongator subcomplex Elp4-6
- PMID: 22556426
- PMCID: PMC3375571
- DOI: 10.1074/jbc.M112.341560
Crystal structure of elongator subcomplex Elp4-6
Abstract
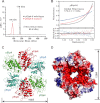
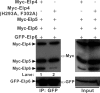
Elongator is a multiprotein complex composed of two subcomplexes, Elp1-3 and Elp4-6. Elongator is highly conserved between yeast and humans and plays an important role in RNA polymerase II-mediated transcriptional elongation and many other processes, including cytoskeleton organization, exocytosis, and tRNA modification. Here, we determined the crystal structure of the Elp4-6 subcomplex of yeast. The overall structure of Elp4-6 revealed that Elp6 acts as a bridge to assemble Elp4 and Elp5. Detailed structural and sequence analyses revealed that each subunit in the Elp4-6 subcomplex forms a RecA-ATPase-like fold, although it lacks the key sequence signature of ATPases. Site-directed mutagenesis and biochemical analyses indicated that the Elp4-6 subcomplex can assemble into a hexameric ring-shaped structure in vitro and in vivo. Furthermore, GST pulldown assays showed that the ring-shaped assembly of the Elp4-6 subcomplex is important for its specific histone H3 binding. Our results may shed light on the substrate recognition and assembly of the holo-Elongator complex.
Figures



References
-
- Shilatifard A., Conaway R. C., Conaway J. W. (2003) The RNA polymerase II elongation complex. Annu. Rev. Biochem. 72, 693–715 - PubMed
-
- Selth L. A., Sigurdsson S., Svejstrup J. Q. (2010) Transcript elongation by RNA polymerase II. Annu. Rev. Biochem. 79, 271–293 - PubMed
-
- Li B., Carey M., Workman J. L. (2007) The role of chromatin during transcription. Cell 128, 707–719 - PubMed
-
- Clapier C. R., Cairns B. R. (2009) The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78, 273–304 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

