Lymphocyte modulation with FTY720 improves hemorrhagic shock survival in swine
- PMID: 22558085
- PMCID: PMC3340389
- DOI: 10.1371/journal.pone.0034224
Lymphocyte modulation with FTY720 improves hemorrhagic shock survival in swine
Abstract
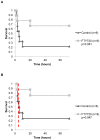
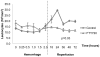
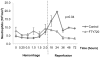
The inflammatory response to severe traumatic injury results in significant morbidity and mortality. Lymphocytes have recently been identified as critical mediators of the early innate immune response to ischemia-reperfusion injury. Experimental manipulation of lymphocytes following hemorrhagic shock may prevent secondary immunologic injury in surgical and trauma patients. The objective of this study is to evaluate the lymphocyte sequestration agent FTY720 as an immunomodulator following experimental hemorrhagic shock in a swine liver injury model. Yorkshire swine were anesthetized and underwent a grade III liver injury with uncontrolled hemorrhage to induce hemorrhagic shock. Experimental groups were treated with a lymphocyte sequestration agent, FTY720, (n = 9) and compared to a vehicle control group (n = 9). Animals were observed over a 3 day survival period after hemorrhage. Circulating total leukocyte and neutrophil counts were measured. Central lymphocytes were evaluated with mesenteric lymph node and spleen immunohistochemistry (IHC) staining for CD3. Lung tissue infiltrating neutrophils were analyzed with myeloperoxidase (MPO) IHC staining. Relevant immune-related gene expression from liver tissue was quantified using RT-PCR. The overall survival was 22.2% in the vehicle control and 66.7% in the FTY720 groups (p = 0.081), and reperfusion survival (period after hemorrhage) was 25% in the vehicle control and 75% in the FTY720 groups (p = 0.047). CD3(+) lymphocytes were significantly increased in mesenteric lymph nodes and spleen in the FTY720 group compared to vehicle control, indicating central lymphocyte sequestration. Lymphocyte disruption significantly decreased circulating and lung tissue infiltrating neutrophils, and decreased expression of liver immune-related gene expression in the FTY720 treated group. There were no observed infectious or wound healing complications. Lymphocyte sequestration with FTY720 improves survival in experimental hemorrhagic shock using a porcine liver injury model. These results support a novel and clinically relevant lymphocyte immunomodulation strategy to ameliorate secondary immune injury in hemorrhagic shock.
Conflict of interest statement
Figures





References
-
- Moore FA, Moore EE. Postinjury multiple organ failure. In: Moore EE, Feliciano DV, Mattox KL, editors. New York: McGraw-Hill; 2004.
-
- De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, et al. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. - PubMed
-
- Spolarics Z, Siddiqi M, Siegel JH, Garcia ZC, Stein DS, et al. Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med. 2003;31:1722–1729. - PubMed
-
- Bandyopadhyay G, De A, Laudanski K, Li F, Lentz C, et al. Negative signaling contributes to T-cell anergy in trauma patients. Crit Care Med. 2007;35:794–801. - PubMed
-
- Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

