Exploring the transcriptome of ciliated cells using in silico dissection of human tissues
- PMID: 22558177
- PMCID: PMC3338421
- DOI: 10.1371/journal.pone.0035618
Exploring the transcriptome of ciliated cells using in silico dissection of human tissues
Abstract
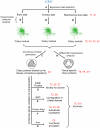
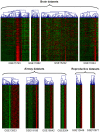
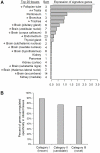
Cilia are cell organelles that play important roles in cell motility, sensory and developmental functions and are involved in a range of human diseases, known as ciliopathies. Here, we search for novel human genes related to cilia using a strategy that exploits the previously reported tendency of cell type-specific genes to be coexpressed in the transcriptome of complex tissues. Gene coexpression networks were constructed using the noise-resistant WGCNA algorithm in 12 publicly available microarray datasets from human tissues rich in motile cilia: airways, fallopian tubes and brain. A cilia-related coexpression module was detected in 10 out of the 12 datasets. A consensus analysis of this module's gene composition recapitulated 297 known and predicted 74 novel cilia-related genes. 82% of the novel candidates were supported by tissue-specificity expression data from GEO and/or proteomic data from the Human Protein Atlas. The novel findings included a set of genes (DCDC2, DYX1C1, KIAA0319) related to a neurological disease dyslexia suggesting their potential involvement in ciliary functions. Furthermore, we searched for differences in gene composition of the ciliary module between the tissues. A multidrug-and-toxin extrusion transporter MATE2 (SLC47A2) was found as a brain-specific central gene in the ciliary module. We confirm the localization of MATE2 in cilia by immunofluorescence staining using MDCK cells as a model. While MATE2 has previously gained attention as a pharmacologically relevant transporter, its potential relation to cilia is suggested for the first time. Taken together, our large-scale analysis of gene coexpression networks identifies novel genes related to human cell cilia.
Conflict of interest statement
Figures



References
-
- Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. - PubMed
-
- Ibanez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet 12 Spec No. 2003;1:R27–35. - PubMed
-
- Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

