Deep and clear optical imaging of thick inhomogeneous samples
- PMID: 22558226
- PMCID: PMC3338470
- DOI: 10.1371/journal.pone.0035795
Deep and clear optical imaging of thick inhomogeneous samples
Abstract
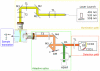
Inhomogeneity in thick biological specimens results in poor imaging by light microscopy, which deteriorates as the focal plane moves deeper into the specimen. Here, we have combined selective plane illumination microscopy (SPIM) with wavefront sensor adaptive optics (wao). Our waoSPIM is based on a direct wavefront measure using a Hartmann-Shack wavefront sensor and fluorescent beads as point source emitters. We demonstrate the use of this waoSPIM method to correct distortions in three-dimensional biological imaging and to improve the quality of images from deep within thick inhomogeneous samples.
Conflict of interest statement
Figures



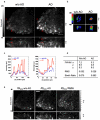
 is
is  mapping images calculated from images obtained with AO and
mapping images calculated from images obtained with AO and  is
is  mapping images calculated from images obtained without AO.
mapping images calculated from images obtained without AO.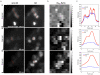
References
-
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. - PubMed
-
- Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EHK. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007. - PubMed
-
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:6006. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

