Deletion of Dicer in smooth muscle affects voiding pattern and reduces detrusor contractility and neuroeffector transmission
- PMID: 22558254
- PMCID: PMC3338793
- DOI: 10.1371/journal.pone.0035882
Deletion of Dicer in smooth muscle affects voiding pattern and reduces detrusor contractility and neuroeffector transmission
Abstract
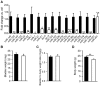
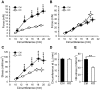
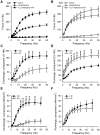
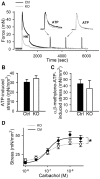
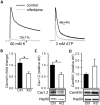
MicroRNAs have emerged as important regulators of smooth muscle phenotype and may play important roles in pathogenesis of various smooth muscle related disease states. The aim of this study was to investigate the role of miRNAs for urinary bladder function. We used an inducible and smooth muscle specific Dicer knockout (KO) mouse which resulted in significantly reduced levels of miRNAs, including miR-145, miR-143, miR-22, miR125b-5p and miR-27a, from detrusor preparations without mucosa. Deletion of Dicer resulted in a disturbed micturition pattern in vivo and reduced depolarization-induced pressure development in the isolated detrusor. Furthermore, electrical field stimulation revealed a decreased cholinergic but maintained purinergic component of neurogenic activation in Dicer KO bladder strips. The ultrastructure of detrusor smooth muscle cells was well maintained, and the density of nerve terminals was similar. Western blotting demonstrated reduced contents of calponin and desmin. Smooth muscle α-actin, SM22α and myocardin were unchanged. Activation of strips with exogenous agonists showed that depolarization-induced contraction was preferentially reduced; ATP- and calyculin A-induced contractions were unchanged. Quantitative real time PCR and western blotting demonstrated reduced expression of Cav1.2 (Cacna1c). It is concluded that smooth muscle miRNAs play an important role for detrusor contractility and voiding pattern of unrestrained mice. This is mediated in part via effects on expression of smooth muscle differentiation markers and L-type Ca(2+) channels in the detrusor.
Conflict of interest statement
Figures



References
-
- Andersson KE. Handb Exp Pharmacol; 2011. Muscarinic acetylcholine receptors in the urinary tract. pp. 319–344. - PubMed
-
- Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci. 2010;154:3–13. - PubMed
-
- Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995;24:159–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

