The HIV matrix protein p17 subverts nuclear receptors expression and induces a STAT1-dependent proinflammatory phenotype in monocytes
- PMID: 22558273
- PMCID: PMC3340403
- DOI: 10.1371/journal.pone.0035924
The HIV matrix protein p17 subverts nuclear receptors expression and induces a STAT1-dependent proinflammatory phenotype in monocytes
Abstract
Background: Long-term remission of HIV-1 disease can be readily achieved by combinations of highly effective antiretroviral therapy (HAART). However, a residual persistent immune activation caused by circulating non infectious particles or viral proteins is observed under HAART and might contribute to an higher risk of non-AIDS pathologies and death in HIV infected persons. A sustained immune activation supports lipid dysmetabolism and increased risk for development of accelerated atehrosclerosis and ischemic complication in virologically suppressed HIV-infected persons receiving HAART.
Aim: While several HIV proteins have been identified and characterized for their ability to maintain immune activation, the role of HIV-p17, a matrix protein involved in the viral replication, is still undefined.
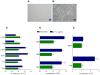
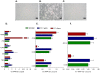
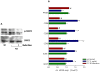
Results: Here, we report that exposure of macrophages to recombinant human p17 induces the expression of proinflammatory and proatherogenic genes (MCP-1, ICAM-1, CD40, CD86 and CD36) while downregulating the expression of nuclear receptors (FXR and PPARγ) that counter-regulate the proinflammatory response and modulate lipid metabolism in these cells. Exposure of macrophage cell lines to p17 activates a signaling pathway mediated by Rack-1/Jak-1/STAT-1 and causes a promoter-dependent regulation of STAT-1 target genes. These effects are abrogated by sera obtained from HIV-infected persons vaccinated with a p17 peptide. Ligands for FXR and PPARγ counteract the effects of p17.
Conclusions: The results of this study show that HIV p17 highjacks a Rack-1/Jak-1/STAT-1 pathway in macrophages, and that the activation of this pathway leads to a simultaneous dysregulation of immune and metabolic functions. The binding of STAT-1 to specific responsive elements in the promoter of PPARγ and FXR and MCP-1 shifts macrophages toward a pro-atherogenetic phenotype characterized by high levels of expression of the scavenger receptor CD36. The present work identifies p17 as a novel target in HIV therapy and grounds the development of anti-p17 small molecules or vaccines.
Conflict of interest statement
Figures




References
-
- Haffar OK, Popov S, Dubrovsky L, Agostini I, Tang H, et al. Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J Mol Biol. 2000;299:359–368. - PubMed
-
- Fiorentini S, Marini E, Caracciolo S, Caruso A. Functions of the HIV-1 matrix protein p17. New Microbiol. 2006;29:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

