Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease
- PMID: 22558451
- PMCID: PMC3340376
- DOI: 10.1371/journal.pone.0036373
Globotriaosylsphingosine accumulation and not alpha-galactosidase-A deficiency causes endothelial dysfunction in Fabry disease
Abstract
Background: Fabry disease (FD) is caused by a deficiency of the lysosomal enzyme alpha-galactosidase A (GLA) resulting in the accumulation of globotriaosylsphingosine (Gb3) in a variety of tissues. While GLA deficiency was always considered as the fulcrum of the disease, recent attention shifted towards studying the mechanisms through which Gb3 accumulation in vascular cells leads to endothelial dysfunction and eventually multiorgan failure. In addition to the well-described macrovascular disease, FD is also characterized by abnormalities of microvascular function, which have been demonstrated by measurements of myocardial blood flow and coronary flow reserve. To date, the relative importance of Gb3 accumulation versus GLA deficiency in causing endothelial dysfunction is not fully understood; furthermore, its differential effects on cardiac micro- and macrovascular endothelial cells are not known.
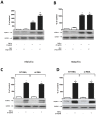
Methods and results: In order to assess the effects of Gb3 accumulation versus GLA deficiency, human macro- and microvascular cardiac endothelial cells (ECs) were incubated with Gb3 or silenced by siRNA to GLA. Gb3 loading caused deregulation of several key endothelial pathways such as eNOS, iNOS, COX-1 and COX-2, while GLA silencing showed no effects. Cardiac microvascular ECs showed a greater susceptibility to Gb3 loading as compared to macrovascular ECs.
Conclusions: Deregulation of key endothelial pathways as observed in FD vasculopathy is likely caused by intracellular Gb3 accumulation rather than deficiency of GLA. Human microvascular ECs, as opposed to macrovascular ECs, seem to be affected earlier and more severely by Gb3 accumulation and this notion may prove fundamental for future progresses in early diagnosis and management of FD patients.
Conflict of interest statement
Figures




References
-
- Zarate YA, Hopkin RJ. Fabry's disease. Lancet. 2008;372:1427–1435. - PubMed
-
- Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, et al. Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis. 2005;28:563–573. - PubMed
-
- Wang RY, Lelis A, Mirocha J, Wilcox WR. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med. 2007;9:34–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

