Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change
- PMID: 22558514
- PMCID: PMC3342746
- DOI: 10.4103/2045-8932.94817
Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change
Abstract
Experimental data indicate that the adventitial compartment of blood vessels, in both the pulmonary and systemic circulations, like the connective tissue stroma in tissues throughout the body, is a critical regulator of vessel wall function in health and disease. It is clear that adventitial cells, and in particular the adventitial fibroblast, are activated early following vascular injury, and play essential roles in regulating vascular wall structure and function through production of chemokines, cytokines, growth factors, and reactive oxygen species (ROS). The recognition of the ability of these cells to generate and maintain inflammatory responses within the vessel wall provides insight into why vascular inflammatory responses, in certain situations, fail to resolve. It is also clear that the activated adventitial fibroblast plays an important role in regulating vasa vasorum growth, which can contribute to ongoing vascular remodeling by acting as a conduit for delivery of inflammatory and progenitor cells. These functions of the fibroblast clearly support the idea that targeting chemokine, cytokine, adhesion molecule, and growth factor production in activated fibroblasts could be helpful in abrogating vascular inflammatory responses and thus in ameliorating vascular disease. Further, the recent observations that fibroblasts in vascular and fibrotic diseases may maintain their activated state through epigenetic alterations in key inflammatory and pro-fibrotic genes suggests that current therapies used to treat pulmonary hypertension may not be sufficient to induce apoptosis or to inhibit key inflammatory signaling pathways in these fibroblasts. New therapies targeted at reversing changes in the acetylation or methylation status of key transcriptional networks may be needed. At present, therapies specifically targeting abnormalities of histone deacytelase (HDAC) activity in fibroblast-like cells appear to hold promise.
Keywords: adventitial; epigenetics; fibroblasts; pulmonary hypertension.
Conflict of interest statement
Figures



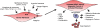

References
-
- Di Wang H, Ratsep MT, Chapman A, Boyd R. Adventitial fibroblasts in vascular structure and function: The role of oxidative stress and beyond. Can J Physiol Pharmacol. 2010;88:177–86. - PubMed
-
- Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovascular Res. 2007;75:679–89. - PubMed
LinkOut - more resources
Full Text Sources

